Materials of construction: Difference between revisions
No edit summary |
|||
| (13 intermediate revisions by 2 users not shown) | |||
| Line 98: | Line 98: | ||
Uniform corrosion is the most common type of corrosion and is considered the “general wastage of material,” while disregarding other sources of wear. Due to the nature of this type of corrosion, the corrosion rate can be predicted and experimentally determined. Corrosion testing is done to predict the penetration rate in inches per year, and can be expressed by the following equation, |
Uniform corrosion is the most common type of corrosion and is considered the “general wastage of material,” while disregarding other sources of wear. Due to the nature of this type of corrosion, the corrosion rate can be predicted and experimentally determined. Corrosion testing is done to predict the penetration rate in inches per year, and can be expressed by the following equation, |
||
<br> |
<br> |
||
<center><math>ipy = 12w |
<center><math>ipy = {12w \over tAp}</math></center> |
||
<br> |
<br> |
||
where ''w''=mass loss in time ''t'' (lb), ''t''=time (years), ''A''=surface area (ft<sup>2</sup>), ''p''=density of material (lb/ft<sup>3</sup>). Acceptable corrosion rates for carbon and low alloy steels are given in Table 2. For more expensive materials, these corrosion rates should be cut in half (Towler et al., 2013). |
where ''w''=mass loss in time ''t'' (lb), ''t''=time (years), ''A''=surface area (ft<sup>2</sup>), ''p''=density of material (lb/ft<sup>3</sup>). Acceptable corrosion rates for carbon and low alloy steels are given in Table 2. For more expensive materials, these corrosion rates should be cut in half (Towler et al., 2013). |
||
| Line 134: | Line 134: | ||
Galvanic corrosion is caused by the contact of dissimilar metals in the presence of an electrically conducting solution. This is caused by flow of current from the more active, anodic metal to the less active cathodic metal. See Figure 3 for the galvanic series of various metals. This increases the corrosion rate, which is dependent on the relative exposed areas of the two metals. In order to minimize this corrosion rate, we should maximize the ratio of cathode to anode area. Another preventative measure is to electrically insulate the two metals in an attempt to break the conducting circuit (Perry et al., 2008). |
Galvanic corrosion is caused by the contact of dissimilar metals in the presence of an electrically conducting solution. This is caused by flow of current from the more active, anodic metal to the less active cathodic metal. See Figure 3 for the galvanic series of various metals. This increases the corrosion rate, which is dependent on the relative exposed areas of the two metals. In order to minimize this corrosion rate, we should maximize the ratio of cathode to anode area. Another preventative measure is to electrically insulate the two metals in an attempt to break the conducting circuit (Perry et al., 2008). |
||
[[File:Galvanic_Series2.png|frame|center|border|'''Figure 3:''' Practical galvanic series of metals and alloys ( |
[[File:Galvanic_Series2.png|frame|center|border|'''Figure 3:''' Practical galvanic series of metals and alloys (Source: www.ssina.com)]] |
||
Pitting is a highly localized form of corrosion, resulting in the development of cavities or pits. This corrosion is much less predictable, and usually occurs in metals where the composition is not uniform. Pitting can be reduced with the use of a surface finish. |
Pitting is a highly localized form of corrosion, resulting in the development of cavities or pits. This corrosion is much less predictable, and usually occurs in metals where the composition is not uniform. Pitting can be reduced with the use of a surface finish. |
||
| Line 150: | Line 150: | ||
Hydrogen embrittlement occurs at elevated temperatures and is the absorption and reaction of hydrogen in a metal resulting in the formation of methane. This results in the loss of ductility and cracking of the metal as decarburization occurs. Hydrogen attack is important to consider when specifying steels for use in hydrogen reforming plants. Alloy steels tend to have a greater resistance than plain carbon steels, but below 500 C, plain carbon steel can be used with limited hydrogen attack. Accepted limits for the use of carbon and low-alloy steels are shown in Nelson curves (Figure 4) (Towler et al., 2013). |
Hydrogen embrittlement occurs at elevated temperatures and is the absorption and reaction of hydrogen in a metal resulting in the formation of methane. This results in the loss of ductility and cracking of the metal as decarburization occurs. Hydrogen attack is important to consider when specifying steels for use in hydrogen reforming plants. Alloy steels tend to have a greater resistance than plain carbon steels, but below 500 C, plain carbon steel can be used with limited hydrogen attack. Accepted limits for the use of carbon and low-alloy steels are shown in Nelson curves (Figure 4) (Towler et al., 2013). |
||
[[File:Nelson_Curves.gif|frame|center|border|'''Figure 4:''' Accepted limits for the use of carbon and low-alloy steels at different hydrogen partial pressures ( |
[[File:Nelson_Curves.gif|frame|center|border|'''Figure 4:''' Accepted limits for the use of carbon and low-alloy steels at different hydrogen partial pressures (Source: www.corrosionclinic.com)]] |
||
==Cycling== |
==Cycling== |
||
| Line 159: | Line 159: | ||
Carbon steel and stainless steels are some of the most common metals used in construction. Carbon steel is an alloy between carbon and iron. Also known as mild steel, carbon steel is one of the most commonly used engineering materials. It is favored because it is relatively cheap and widely available. It also has good tensile strength and ductility. However, carbon steel is not generally resistant to corrosion which can be an issue in many environments. When corrosion is expected, stainless steel is often favored. Stainless steel, especially with higher levels of chromium, is more resistant to corrosion (Towler et al., 2013). Stainless steel is also a better choice for low temperatures, as it has a minimum rating of -425 F, as opposed to the minimum rating of carbon steel of -50 F. Stainless steels are also a better choice than carbon steel when temperatures above 1000 F are expected (Biegler et al., 1997) |
Carbon steel and stainless steels are some of the most common metals used in construction. Carbon steel is an alloy between carbon and iron. Also known as mild steel, carbon steel is one of the most commonly used engineering materials. It is favored because it is relatively cheap and widely available. It also has good tensile strength and ductility. However, carbon steel is not generally resistant to corrosion which can be an issue in many environments. When corrosion is expected, stainless steel is often favored. Stainless steel, especially with higher levels of chromium, is more resistant to corrosion (Towler et al., 2013). Stainless steel is also a better choice for low temperatures, as it has a minimum rating of -425 F, as opposed to the minimum rating of carbon steel of -50 F. Stainless steels are also a better choice than carbon steel when temperatures above 1000 F are expected (Biegler et al., 1997) |
||
Other options include nickel and alloys, including Monel, a nickel-copper alloy (Ulrich, 1984). These are also corrosion-resistant to sulfuric and hydrochloric acids and salt water. Nickel-chromium alloys are good to chemical resistance at high temperatures (Turton et al., 2012). Copper and alloys have the advantage of corrosion resistance and good thermal conductivity. Thus, copper is often favored for heat transfer equipment (Ulrich, 1984). Aluminum and its alloys are more moderately priced than copper metals, are lightweight, and are better for low temperatures than carbon steel, however, they have lower strength |
Other options include nickel and alloys, including Monel, a nickel-copper alloy (Ulrich, 1984). These are also corrosion-resistant to sulfuric and hydrochloric acids and salt water. Nickel-chromium alloys are good to chemical resistance at high temperatures (Turton et al., 2012). Copper and alloys have the advantage of corrosion resistance and good thermal conductivity. Thus, copper is often favored for heat transfer equipment (Ulrich, 1984). Aluminum and its alloys are more moderately priced than copper metals, are lightweight, and are better for low temperatures than carbon steel, however, they have lower strength (Ulrich, 1984). |
||
<center> |
<center> |
||
{| class="wikitable" |
{| class="wikitable" |
||
| Line 169: | Line 169: | ||
! Brinell Hardness |
! Brinell Hardness |
||
! Specific Gravity |
! Specific Gravity |
||
! Max Allowable Stress ( |
! Max Allowable Stress (N/mm<sup>2</sup>) |
||
! Price ($/kg) (2010) |
|||
! Relative Cost |
|||
! Relative Cost (2010) |
|||
|- |
|- |
||
| Mild (carbon) steel |
| Mild (carbon) steel |
||
| Line 177: | Line 178: | ||
| 100-200 |
| 100-200 |
||
| 7.9 |
| 7.9 |
||
| |
| 0.02 |
||
| 0.82 |
|||
| 1 |
| 1 |
||
|- |
|- |
||
| Line 185: | Line 187: | ||
| 160 |
| 160 |
||
| 8.0 |
| 8.0 |
||
| |
| 0.14 |
||
| 2.55-3.79 |
|||
| 2.0-3.0 |
| 2.0-3.0 |
||
|- |
|- |
||
| Line 193: | Line 196: | ||
| 30-100 |
| 30-100 |
||
| 8.9 |
| 8.9 |
||
| |
| 0.046 |
||
| 8.44 |
|||
| 22.8 |
| 22.8 |
||
|- |
|- |
||
| Line 201: | Line 205: | ||
| 80-150 |
| 80-150 |
||
| 8.9 |
| 8.9 |
||
| |
| 0.069 |
||
| 21.74 |
|||
| 39.2 |
| 39.2 |
||
|- |
|- |
||
| Line 209: | Line 214: | ||
| 120-250 |
| 120-250 |
||
| 8.8 |
| 8.8 |
||
| |
| 0.13 |
||
| 17.11 |
|||
| 16.4 |
| 16.4 |
||
|- |
|- |
||
| Line 217: | Line 223: | ||
| 150 |
| 150 |
||
| 4.5 |
| 4.5 |
||
| |
| 0.069 |
||
| 7.39 |
|||
| 6.8 |
| 6.8 |
||
|} |
|} |
||
| Line 226: | Line 233: | ||
<center><math> Cost Rating = {c * \rho \over {\sigma_d}} </math> </center> |
<center><math> Cost Rating = {c * \rho \over {\sigma_d}} </math> </center> |
||
Where '''c''' is cost per unit mass ($/kg), '''ρ''' is density (kg/m3), and ''' |
Where '''c''' is cost per unit mass ($/kg), '''ρ''' is density (kg/m3), and '''σ<sub>d</sub>''' is maximum allowable stress (N/mm2). |
||
This rating lets designers find what material will be the cheapest for the requirements needed for the design. More variables can be inserted in the equation depending on the design’s restraints (e.g. maximum temperature rating). As such, the rating is somewhat limited. |
This rating lets designers find what material will be the cheapest for the requirements needed for the design. More variables can be inserted in the equation depending on the design’s restraints (e.g. maximum temperature rating). As such, the rating is somewhat limited. |
||
| Line 341: | Line 348: | ||
Type 300 series are more commonly used in chemical processes for their excellent corrosion resistance. Type 304, or 18/8 as its known for its 18% chromium and 8% nickel content, uses the minimum amount of chromium and nickel to still have its stable austenitic structure. This keeps its price low while still having excellent properties. The carbon content is usually low enough to prevent weld decay making the lack of potential heat treatment not a problem. Type 304L has even less carbon that type 304 (less than 0.03%) and is usually used in areas where carbide precipitation would occur for type 304 (e.g. thicker welded sections). For high temperature use, there is type 321, which is more temperature resistant and is a stabilized version of type 304. Types 309 and 310 can prevent oxidation at high temperatures due to increased chromium content. For even more corrosion resistance, type 316 is the best for its added molybdenum, which can thwart corrosion from dilute sulfuric acid (Towler et al., 2013). |
Type 300 series are more commonly used in chemical processes for their excellent corrosion resistance. Type 304, or 18/8 as its known for its 18% chromium and 8% nickel content, uses the minimum amount of chromium and nickel to still have its stable austenitic structure. This keeps its price low while still having excellent properties. The carbon content is usually low enough to prevent weld decay making the lack of potential heat treatment not a problem. Type 304L has even less carbon that type 304 (less than 0.03%) and is usually used in areas where carbide precipitation would occur for type 304 (e.g. thicker welded sections). For high temperature use, there is type 321, which is more temperature resistant and is a stabilized version of type 304. Types 309 and 310 can prevent oxidation at high temperatures due to increased chromium content. For even more corrosion resistance, type 316 is the best for its added molybdenum, which can thwart corrosion from dilute sulfuric acid (Towler et al., 2013). |
||
===Nickel, Copper, and Monel=== |
|||
Nickel, by itself, has unique inherent advantages to other materials. Unlike stainless steel, nickel is easily workable and not subject to corrosion cracking. This is due nickel's slow rate of oxidation at room temperature. Nickel is typically used in equipment that handle caustic alkalis at temperatures above 70 <sup>o</sup>C. This is because that is the limit at which carbon steels can operate, thus Nickel acts as a strong replacement (Towler et al., 2013). |
|||
Nickel is typically used in processing equipment as it is particularly good at maintaining product purity. These products can range from food to synthetic fibers. It is rare in chemical processes to use pure nickel as making a nickel alloy is a very easy process and can greatly improve certain material properties. Nickel has complete solid solubility with copper, which makes forming alloys with copper simple (Ulrich, 1984). |
|||
Copper is a soft metal which is equally easy to work with as Nickel and is found in abundant supply. Its soft nature provides it with good electrical and thermal conductivity. Copper is common in the food industry as well, typically in brewing. Transfer tubes and small-bore pipes often contain copper for its strong heat transfer properties. Copper doesn't react with water but it does slowly react with oxygen in the air. This reaction doesn't produce rust but rather a layer of copper dioxide which protects copper from corrosion. Copper is resistant to caustic alkalis, except ammonia, and many salts and organic acids. Mineral acids are harmful to copper, however (Towler et al., 2013). |
|||
Copper can form many alloys. The main ones are brasses (alloyed with zinc) and bronzes (alloyed with tin). They are nearly equally corrosion resistant to metals. Their main use is in valves and other small fittings along with pipes (Towler et al., 2013). As temperature goes down, copper alloys become more ductile and even stronger. An alloy with 70% copper that contains nickel and other strengthening elements is called a "cupronickel" alloy. Cupronickels can easily be fabricated due to the complete solubility between copper and nickel. Cupronickels are strong against seawater corrosion and are resistant to macrofouling and thus are often used in marine applications (e.g. seawater desalination plants, offshore oil platforms, etc). Choosing the right alloy depends on what properties one would like most expressed from thermal conductivity to tensile strength (CDAI). |
|||
<center> |
|||
{| class="wikitable" style="text-align:center" |
|||
|+ '''Table 5:''' Typical Physical Properties of Copper Nickel Alloys (Copper Development Association Inc.) |
|||
! Alloy |
|||
!Density<br/>g/cm<sup>3</sup> |
|||
!Thermal conductivity<br/>W/(m·K) |
|||
!Thermal expansion<br/>µm/(m·K) |
|||
!Electrical resistivity<br/>µOhm·cm |
|||
!Elastic modulus<br/>GPa |
|||
!Yield strength<br/>MPa |
|||
!Tensile strength<br/>MPa |
|||
|- |
|||
| 90-10 |
|||
|8.9 |
|||
|40 |
|||
|17 |
|||
|19 |
|||
|135 |
|||
| 105 |
|||
| 275 |
|||
|- |
|||
| 70-30 |
|||
|8.95 |
|||
|29 |
|||
|16 |
|||
|34 |
|||
|152 |
|||
| 125 |
|||
| 360 |
|||
|- |
|||
| 66-30-2-2 |
|||
|8.86 |
|||
|25 |
|||
|15.5 |
|||
|50 |
|||
|156 |
|||
| 170 |
|||
| 435 |
|||
|} |
|||
</center> |
|||
Monel is a nickel alloy compromised of mostly nickel (~65%) and copper with other strengthening elements like iron. Monel is stronger than pure nickel and is considered a "single-phase alloy" due to the compatibility of nickel and copper. It is the second most commonly used alloy in chemical plants and is right up there with stainless steel in terms of its widespread uses. It also has specific advantages in certain situations when compared with stainless steels, such as when working with dilute mineral acids and working under reducing conditions. Its only big negative is its price when compared with steel (Towler et al., 2013). Monel is very difficult to machine due to work-hardening which makes its production cost increase relative to its material cost (Monel can be up to 10 times more expensive than nickel and copper). Monel is therefore typically used in situations where it has its inherent advantages and where its cost is outweighed by its benefits. Monel is used in many of the same industries as cupronickel alloys, including marine engineering and oil refinery. However, one must be careful not to put monel near steel in seawater due to the potential of galvanic corrosion (Ulrich, 1984). |
|||
===Aluminum Alloys and Titanium=== |
|||
Pure aluminum is highly resistant to corrosion, more so than its alloys. It comes from its formation of a thin oxide film, similar to stainless steel. However it lacks mechanical strength which is why pure aluminum is rarely used. The "Duralumin" or "Dural" range of aluminum alloys are most common with small percentages of copper and magnesium (<5%). These alloys are corrosion resistant and strong. They are used in the textile and food industries in places where mild steel may cause contamination (Towler et al., 2013). Aluminum is often appropriate for cryogenic operations (Turton et al., 2012). |
|||
Titanium is growing in use in the chemical industry due to its resistance to seawater corrosion and chlorine cracking. It isn't as strong as stainless steel for holding loads but it can withstand more strain which may be of use in liquid oxidation processes. Its thermal conductivity properties make it a good replacement for cupronickel (Towler et al., 2013). |
|||
==Plastics== |
==Plastics== |
||
| Line 347: | Line 411: | ||
{| class="wikitable" |
{| class="wikitable" |
||
|- |
|- |
||
|+ '''Table |
|+ '''Table 6:''' Mechanical Properties and Relative Costs of Polymers (Towler et al., 2013) |
||
! Material |
! Material |
||
! Tensile Strength (N/mm^2) |
! Tensile Strength (N/mm^2) |
||
| Line 391: | Line 455: | ||
=Case Study= |
=Case Study= |
||
==Example 1== |
|||
'''Example Problem''': |
|||
Chlorobenzene is produced by reacting liquid benzene with gaseous chlorine. The reaction takes place at 328K and 2.4 bar. If both the feeds are at 293K and atmospheric pressure, what are appropriate materials for the inlet piping and the reactor? (Adapted from ''Chemical Engineering Design'' (Towler et al., 2013)). |
Chlorobenzene is produced by reacting liquid benzene with gaseous chlorine. The reaction takes place at 328K and 2.4 bar. If both the feeds are at 293K and atmospheric pressure, what are appropriate materials for the inlet piping and the reactor? (Adapted from ''Chemical Engineering Design'' (Towler et al., 2013)). |
||
Both benzene and dry chlorine are not corrosive and therefore, carbon steel can be used as the inlet piping. Note that if the gaseous chlorine is actually wet chlorine, it becomes very corrosive to most metals and a plastic should likely be used. While the reactor is at higher pressure than atmospheric pressure, it is well below the maximum allowable stress for all common materials (Towler et al., 2013). However, a side product of the reactor is HCl which is corrosive. Likely, the HCl concentration will not be high enough to corrode the material but this should be investigated further. If concentration is >50% either in the reactor or later in the process, another material such as a plastic should be |
Both benzene and dry chlorine are not corrosive and therefore, carbon steel can be used as the inlet piping. Note that if the gaseous chlorine is actually wet chlorine, it becomes very corrosive to most metals and a plastic should likely be used. While the reactor is at higher pressure than atmospheric pressure, it is well below the maximum allowable stress for all common materials (Towler et al., 2013). However, a side product of the reactor is HCl which is corrosive. Likely, the HCl concentration will not be high enough to corrode the material but this should be investigated further. If concentration is >50% either in the reactor or later in the process, another material such as a plastic should be |
||
==Example 2== |
|||
Corrosion must be taken into account while designing a pressure vessel. Equipment thickness must be able to handle the wear and tear of plant operation. In general, corrosion allowances are about 1.5-5mm thick (Towler et al., 2013). |
|||
'''Example Problem''': |
|||
Consider a packed bed catalytic reactor for the reaction of propylene with hydrogen peroxide to form propylene oxide. The propylene feed rate is 10,000 lb/h at 60C. The reactor is isothermal and operates at 500 psi. The catalyst possesses a surface density of 0.25 sites/nm<sup>2</sup>, a particle surface area of 800 m<sup>2</sup>/g, a particle density of 1 g/cm<sup>3</sup>, a diameter of 250 micrometers, and a void fraction of 0.4. The reactor volume is 1.16 m<sup>3</sup>, with a diameter of 1 m, and length of 2 m. There is an allowance of 0.5 m on each end of the catalyst bed for internals. What is the estimated wall thickness? |
|||
The design pressure of this pressure vessel will be 550 psi, with a design temperature of 88 C. Due to the corrosive media of hydrogen peroxide, stainless steel should be used, and the corrosion allowance is 4 mm. However, hoop stress and longitudinal stress needs to be taken into consideration to determine the necessary thickness of the pipes. |
|||
Hoop stress can be calculated by the equation |
|||
<center><math>t = {Pi Di \over {2SE - 1.2Pi}}</math></center> |
|||
''Pi'' = internal pressure, ''D'' = internal diameter, ''S'' = maximum allowable stress (found in literature tables), ''E'' = welded joint efficiency (based on type of weld). In this example, hoop stress is 15.3 mm. |
|||
Longitudinal stress can be calculated by the equation |
|||
<center><math>t = {Pi Di \over {4SE + 0.8Pi}}</math></center> |
|||
Here the longitudinal stress is 7.5 mm |
|||
Hoop stress dominates as it is greater than the calculated longitudinal stress thickness, so the hoop stress added on with the corrosion allowance = 15.3 mm + 4.00 mm = 19.3 mm. Therefore, the wall thickness should be 19.3 mm. |
|||
==Example 3== |
|||
'''Example Problem''': |
|||
Chemical Corporation X (CCX) is known for their highly efficient and cost-effective production pathway for producing ethylene. Due to pressures from newer competitors in the market, the research and development team decides to find ways to cut cost . A researcher discovers a new material he names Material Y. It has a density of 16000 kg/m<sup>3</sup> s cost of production is $2.00/kg. Its maximum allowable stress is 0.14 N/mm<sup>2</sup>, which is the same as the stainless steel grade CCX uses for its now aging storage tanks. Would it be worth it for CCX to use this new Material Y in place of purchasing their usual stainless steel grade material for its storage tanks? The stainless steel is being purchased at $3.30/kg currently and its density is 8000 kg/m<sup>3</sup>. |
|||
Without much more information to go by, one can only make initial cost comparisons. This is what the cost rating equation is useful for. Using the equation below (Towler et al., 2013): |
|||
<center><math> Cost Rating = {c * \rho \over {\sigma_d}} </math> </center> |
|||
Inputing the information from the example results in a cost rating of 188,571 for stainless steel and a cost rating of 228,571 for Material Y. Material Y is roughly 1.2 times as expensive stainless steel when comparing the relative cost ratings despite Material Y's lower price per kilogram. CCX should stick with their current stainless steel grade for their storage tanks. |
|||
=References= |
=References= |
||
| Line 401: | Line 499: | ||
Callister W, Rethwisch D. Materials Science and Engineering. Wiley: New York, 2011. |
Callister W, Rethwisch D. Materials Science and Engineering. Wiley: New York, 2011. |
||
Copper Development Association Inc. Copper.org [Internet]. Physical Properties of Copper Nickel Alloys [2000]. Available from: http://www.copper.org/applications/marine/cuni/properties/physical/ |
|||
Engineering Archives Website. Stress Strain Diagram. http://www.engineeringarchives.com/les_mom_stressstraindiagram.html. |
Engineering Archives Website. Stress Strain Diagram. http://www.engineeringarchives.com/les_mom_stressstraindiagram.html. |
||
| Line 406: | Line 506: | ||
Peters MS, Timmerhaus KD. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw Hill; 2003. |
Peters MS, Timmerhaus KD. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw Hill; 2003. |
||
Stainless Steel Industry of America. The Stainless Steel Information Center [Internet]. Relationship between hardness and strength [February 2013]. Available from: |
|||
http://www.ssina.com/overview/history.html |
|||
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013. |
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013. |
||
| Line 414: | Line 517: | ||
VanAken D. Industrial Heating Website [Internet]. Relationship between hardness and strength [updated 2001 Mar 7; cited 2015 Mar 1]. Available from: http://www.industrialheating.com/articles/84495-engineering-concepts-relationship-between-hardness-and-strength?v=preview. |
VanAken D. Industrial Heating Website [Internet]. Relationship between hardness and strength [updated 2001 Mar 7; cited 2015 Mar 1]. Available from: http://www.industrialheating.com/articles/84495-engineering-concepts-relationship-between-hardness-and-strength?v=preview. |
||
Stainless Steel Industry of America. The Stainless Steel Information Center [Internet]. Relationship between hardness and strength [February 2013]. Available from: |
|||
http://www.ssina.com/overview/history.html |
|||
Latest revision as of 23:58, 21 February 2016
Author: Katie Johnson [2015] , Helen Wu [2016] , Hassan Ali [2016]
Stewards: Jian Gong and Fengqi You
Material Properties
There are several properties of a material that can affect its suitability for the design. Before choosing a material, the designer should be aware of the following properties. Note that these properties for different common materials are often already collected and are available in various forms from manufacturers or in various textbooks.
Tensile Strength
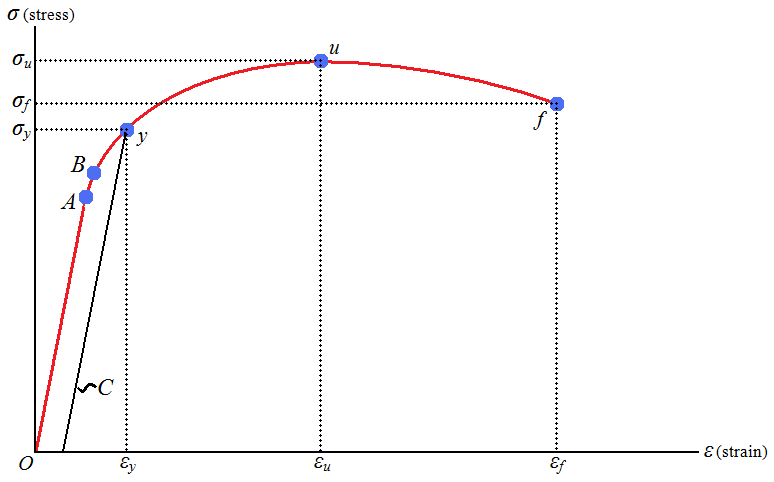
The tensile strength, or tensile stress, of a material is the maximum amount of stress it can withstand before fracture. Proof stress, or yield stress, is similar, but measures that maximum amount of stress a material can withstand before deformation becomes permanent. Figure 1 below demonstrates where tensile strength (point u) and yield stress (point y) lay on the stress-strain curve for a material. There are standard tensile tests that measure tensile strength; however, strength is a common material property that is often already tabulated (Towler et al., 2013).
In addition to considerations such as the pressure of the process, there are often guidelines that specify maximum allowable stress. One such set of guidelines is laid out by ASME in their Boiler and Pressure Vessel Code (Towler et al., 2013). This should be consulted while designing pressure vessels. There are also equations that can estimate these values. The maximum pressure that a cylindrical vessel can withstand is given by the following equations, where t is shell thickness, p is pressure, R is the inside vessel radius, and S is the allowable tensile stress:
There are tabulations of S for various metals found in Perry’s Handbook (Ulrich, 1984).
Modulus of Elasticity
The modulus of elasticity of a material, sometimes called its stiffness, measures the amount a material deforms when a certain amount of stress is placed on it. This measure applies when elastic deformation occurs, that is, when all deformation is reversible and is linearly proportional to stress (Callister et al., 2011). In Figure 1, the modulus of elasticity for the material would apply between the origin and point y. This is important because it measures the resistance of a material to bending and buckling (Towler et al., 2013).
Ductility
Ductility measures the amount a material will deform before it fractures (Towler et al., 2013). The equation for ductility is as follows:
(Callister et al., 2011).
When a material has very low ductility it is defined as brittle. For example, in Figure 1 above, point f will be much closer to point u for a brittle material than for a ductile material. Brittle materials undergo very little deformation before they fracture, which means that in processes, there can be very little warming before a rupture. Some materials have a ductile-brittle transition points at low temperature. While these materials generally exhibit ductile properties, at low enough temperatures, they will not deform and will exhibit brittle fracture (Peters et al., 2003).
Hardness
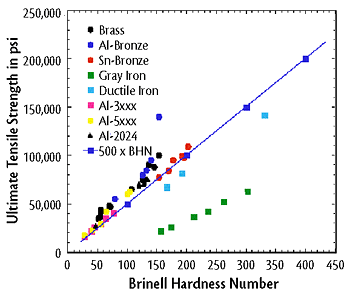
The material’s ability to resist plastic deformation such as dents (Towler et al., 2013). There are many simple and relatively inexpensive tests, such as Rockwell Hardness Tests and Brinell Hardness Tests, which can determine the hardness of a material. It is useful to know the hardness of a material because it can be used to predict other mechanical properties such as tensile strength and can often be easier to determine (Callister et al., 2011). Figure 2 below shows an example of the correlation between Brinell hardness number and tensile strength.
Fatigue Resistance
Fatigue is failure of a material that can occur when there is cyclic loading on equipment, for example, in pumps. It can also occur if there are cycles of temperature or pressure (Towler et al., 2013). When there is cyclic loading, failure can occur at lower stress levels than the normal tensile strength. Fatigue failure is generally very similar to brittle failure with very little plastic deformation (Callister et al., 2011).
Other considerations
There are many other properties to consider while selecting a material. For example, creep is the amount a material deforms while it is under constant tensile stress over long periods of time and can especially be a problem for metals at high temperatures. Other considerations include the ease of fabrication, including welding ability and flexibility, the availability and cost of material, thermal conductivity (which is especially important for equipment like heat exchangers), electrical resistance, and magnetic properties for certain cases (Towler et al., 2013).
Process Considerations
Before choosing a material for a process, basic information must be collected, including temperature, pressure, and chemicals involved. The properties could affect the choice of materials.
Process Temperature and Pressure
In addition to knowledge of the average temperature a process with operate at, the engineer must be aware of the maximum and minimum temperature that could occur. While picking materials, the effect of temperature of material properties must be considered. Higher temperatures generally decrease the tensile strength and elastic modulus of metals (Towler et al., 2013).
Additionally, very low temperatures can cause some materials to brittle fracture. Therefore, if the minimum process temperature is below the minimum allowable temperature for a material, a different material (such as low temperature carbon steel) must be selected. Note that the expected, maximum, and minimum environmental conditions should be considered in addition to internal conditions.
| High temperature service | Low temperature service | ||
|---|---|---|---|
| Tmax (F) | Steel | Tmin (F) | Steel |
| 950 | Carbon steel | -50 | Carbon steel |
| 1300 | 330 stainless steel | -75 | Nickel steel (A203) |
| 1500 | Stainless steels (304,321,347,316) | -320 | Nickel steel (A325) |
| 2000 | Cast stainless, HC | -425 | Stainless steels (302,304,310,347) |
Similarly, the maximum and minimum pressures must be examined in relation to material properties. Different materials have different tensile stresses which affect that maximum pressure that can be used. When internal pressure is less than external pressure (that is, the process operates in a vacuum), either materials with higher allowable tensile stresses should be used or thickness should be increased.
Corrosion
If a process included certain corrosive chemicals such as oxygen, special allocations must be made for the materials. Additionally, environmental conditions such as salt from a nearby ocean should be considered. If corrosion is expected, the engineer should consider this in the material selection. This could involve picking a material that is naturally corrosive-resistant or by coating the inside of the pipe of equipment. These coatings can be made of paint or other organic coatings, especially for resistance to atmospheric corrosion (Towler et al., 2013). For internal protection, materials can be lined with rubber, glass, stainless steel or various polymers (Ulrich, 1984; Turton et al., 2012).
Types of Corrosion
Uniform corrosion is the most common type of corrosion and is considered the “general wastage of material,” while disregarding other sources of wear. Due to the nature of this type of corrosion, the corrosion rate can be predicted and experimentally determined. Corrosion testing is done to predict the penetration rate in inches per year, and can be expressed by the following equation,
where w=mass loss in time t (lb), t=time (years), A=surface area (ft2), p=density of material (lb/ft3). Acceptable corrosion rates for carbon and low alloy steels are given in Table 2. For more expensive materials, these corrosion rates should be cut in half (Towler et al., 2013).
| Corrosion Rate | ||
|---|---|---|
| ipy | mm/y | |
| Completely satisfactory | <0.01 | 0.25 |
| Use with caution | <0.03 | 0.75 |
| Use only for short exposures | <0.6 | 1.5 |
| Completely unsatisfactory | >0.06 | 1.5 |
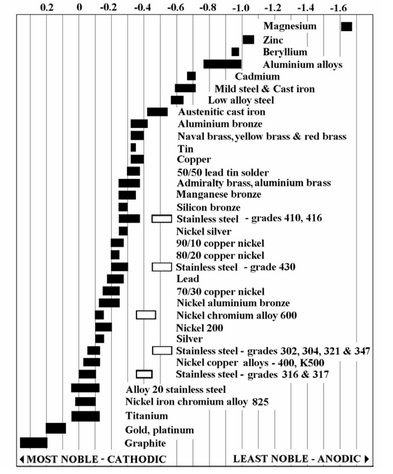
Galvanic corrosion is caused by the contact of dissimilar metals in the presence of an electrically conducting solution. This is caused by flow of current from the more active, anodic metal to the less active cathodic metal. See Figure 3 for the galvanic series of various metals. This increases the corrosion rate, which is dependent on the relative exposed areas of the two metals. In order to minimize this corrosion rate, we should maximize the ratio of cathode to anode area. Another preventative measure is to electrically insulate the two metals in an attempt to break the conducting circuit (Perry et al., 2008).
Pitting is a highly localized form of corrosion, resulting in the development of cavities or pits. This corrosion is much less predictable, and usually occurs in metals where the composition is not uniform. Pitting can be reduced with the use of a surface finish.
Erosion-corrosion can occur if the solution in contact with the metal contains suspended particles, or is of high velocity. In order to account for these factors and the resulting addition erosion, a more resistant material must be used at the surface.
Intergranular corrosion is a selective corrosion, occurring at grain or crystal boundaries of a metal or alloy. Even though the actual loss of material is small, it can cause failure of equipment by causing a loss of strength and ductility. This corrosion is very common with alloys, and rare with pure metals. It is caused by the presence of impurities existing at the grain boundary that accumulate during heat treatment.
Stress corrosion takes into account internal and external stressors that can cause premature failure of the material (Towler et al., 2013). Residual internal stressors include unequal cooling from high temperatures, structural rearrangements involving volume change, and stresses caused by rivets and bolts (Perry et al. 2008). External tensile stresses at the surface, along with the internal stresses, can cause this stress cracking. This is avoided during design by selecting materials that are not vulnerable in the specific corrosion environment and by eliminating high stresses (Towler et al., 2013). The source of stress corrosion is often due to the development of stresses during fabrication and welding, and can cause failure prematurely in as short amount of time as minutes or as long as years.
Liquid-metal corrosion occurs when liquid metals penetrate cracks between grain and weld boundaries. One example is mercury attack on aluminum alloys, and can cause catastrophic failure of the equipment (Perry et al., 2008).
Corrosion can also occur through high temperature oxidation for some low alloy steels. For these materials, the operation temperatures must be below 480 C to prevent oxidation and corrosion. There are ways to prevent this oxidation, such as creating a boundary between the solution and the material. Here, chromium is effective in resisting oxidation by creating an oxide film in between the solution and the equipment. Sulfidation is also a concern. Sulfur is a common element present in oil refining and energy plants. However, sulfur is very corrosive and can attack the equipment causing heavy corrosion.
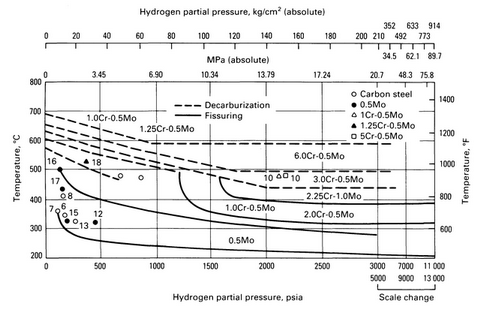
Hydrogen embrittlement occurs at elevated temperatures and is the absorption and reaction of hydrogen in a metal resulting in the formation of methane. This results in the loss of ductility and cracking of the metal as decarburization occurs. Hydrogen attack is important to consider when specifying steels for use in hydrogen reforming plants. Alloy steels tend to have a greater resistance than plain carbon steels, but below 500 C, plain carbon steel can be used with limited hydrogen attack. Accepted limits for the use of carbon and low-alloy steels are shown in Nelson curves (Figure 4) (Towler et al., 2013).
Cycling
Cycling occurs when a certain aspect of a process (temperature, pressure, or material levels) continually cycles between high and low levels. Cycling puts additional stress on the system and should be considered when selecting materials (Callister et al., 2011).
Common Materials
Metals
Carbon steel and stainless steels are some of the most common metals used in construction. Carbon steel is an alloy between carbon and iron. Also known as mild steel, carbon steel is one of the most commonly used engineering materials. It is favored because it is relatively cheap and widely available. It also has good tensile strength and ductility. However, carbon steel is not generally resistant to corrosion which can be an issue in many environments. When corrosion is expected, stainless steel is often favored. Stainless steel, especially with higher levels of chromium, is more resistant to corrosion (Towler et al., 2013). Stainless steel is also a better choice for low temperatures, as it has a minimum rating of -425 F, as opposed to the minimum rating of carbon steel of -50 F. Stainless steels are also a better choice than carbon steel when temperatures above 1000 F are expected (Biegler et al., 1997)
Other options include nickel and alloys, including Monel, a nickel-copper alloy (Ulrich, 1984). These are also corrosion-resistant to sulfuric and hydrochloric acids and salt water. Nickel-chromium alloys are good to chemical resistance at high temperatures (Turton et al., 2012). Copper and alloys have the advantage of corrosion resistance and good thermal conductivity. Thus, copper is often favored for heat transfer equipment (Ulrich, 1984). Aluminum and its alloys are more moderately priced than copper metals, are lightweight, and are better for low temperatures than carbon steel, however, they have lower strength (Ulrich, 1984).
| Material | Tensile strength (N/mm2) | Modulus of elasticity (kN/mm2) | Brinell Hardness | Specific Gravity | Max Allowable Stress (N/mm2) | Price ($/kg) (2010) | Relative Cost (2010) |
|---|---|---|---|---|---|---|---|
| Mild (carbon) steel | 430 | 210 | 100-200 | 7.9 | 0.02 | 0.82 | 1 |
| Stainless steel | >540 | 210 | 160 | 8.0 | 0.14 | 2.55-3.79 | 2.0-3.0 |
| Copper | 200 | 110 | 30-100 | 8.9 | 0.046 | 8.44 | 22.8 |
| Nickel | 500 | 210 | 80-150 | 8.9 | 0.069 | 21.74 | 39.2 |
| Monel | 650 | 170 | 120-250 | 8.8 | 0.13 | 17.11 | 16.4 |
| Titanium | 500 | 110 | 150 | 4.5 | 0.069 | 7.39 | 6.8 |
Material Costs
Relative cost rating (Table 3) is determined by a set equation made with factors that normally determine a materials cost. This equation (Towler et al., 2013) is
Where c is cost per unit mass ($/kg), ρ is density (kg/m3), and σd is maximum allowable stress (N/mm2).
This rating lets designers find what material will be the cheapest for the requirements needed for the design. More variables can be inserted in the equation depending on the design’s restraints (e.g. maximum temperature rating). As such, the rating is somewhat limited.
Materials with higher maximum allowable stress tend to be more expensive but can be used in smaller amounts (Towler et al., 2013).
Stainless Steels
Of all of the major metal types used in chemical design, the most commonly used and studied is stainless steel. Stainless steels are well known for the resistance to corrosion, making them highly applicable in the world of chemical processing wherein stainless steel pipes and tanks are used to contain corrosive liquids. Stainless steel is defined as a steel alloy with a minimum of 10.5% chromium by mass. Like steel, stainless steel comes in a variety of forms that can be made by altering its microstructure and then be further altered by changing the alloy's chemical makeup (i.e. adding or removing its elements). This flexibility of stainless steel allows it to be used in almost any application, depending on the requirements of the design. Generally, however, stainless steel is used when one needs the engineering properties of steel but with the added benefit of preventing corrosion (Biegler et al., 1997). Stainless steels work by containing the minimum necessary level of chromium to form a film of chromium oxide on top of the steel when in the presence of oxygen. This process blocks corrosion from spreading into the internal structure of the steel. Without this passive film creation, stainless steel would rust when exposed oxygen and water much like carbon steel does. However, there is a downside as the added benefit of stainless steel only works in the presence of oxygen. As such, stainless steels are not used in low air environments (SSIA).
Stainless steels come in three major types based on their microstructure: Ferritic, Martensitic, and Austenitic. Ferritic stainless steels have a body-centered cubic structure. They contain a wide range of chromium, anywhere from 13-20%, and no nickel with a maximum of 0.1% carbon. Ferritic stainless steels are usually less expensive and therefore are on the lower end of chromium content. This makes them less resistant to corrosion when compared to other stainless steels, but due to its structure it contains better engineering properties (Towler et al., 2013). Martensitic stainless steels are made from heating and quenching austenitic stainless steels to create a unique microstructure that is harder overall than austenitic stainless steels. This hardness can prevent weld decay, but it also makes the steel very brittle. Often, martensitic stainless steels are tempered to make a hard and tough material. The chromium content is around 10-12% with less than 2% nickel. Due to the low chromium and nickel content, martensitic stainless steels have relatively little corrosion resistance (SSIA).
Austenitic stainless steels make up more than 70% of the stainless steel produced around the world. They are distinguished by their face-centered cubic structure and carbides usage. The addition of nickel into the steel stabilizes the austenitic structure of the iron within. As such, austenitic stainless steels contain a minimum of 7% nickel with 18-20% chromium (Towler et al., 2013). They are popular due to their high corrosion resistance. Austenitic stainless steels cannot harden under heat treatment, which makes them susceptible to weld decay. Rather, they must go through the complicated process of martensitic treatment (SSIA).
There are “grades” of austenitic stainless steels that correspond to a specific chemical composition. The 200 series of austenitic stainless steels are typically more “general purpose” and cheaper as they substitute manganese for nickel. This makes them better rounded but less corrosion resistant. Type 201, for example, can harden under cold treatment (SSIA).
| Specification No. | Composition % | |||||||
|---|---|---|---|---|---|---|---|---|
| AISI No. | C Max | Si Max | Mn Max | Cr Range | Ni Range | Mo Range | Ti | Nb |
| 304 | 0.08 | 2.00 | 17.5-20.0 | 8.0-11.0 | ||||
| 304L | 0.03 | 1.00 | 2.00 | 17.5-20.0 | 8.0-12.0 | |||
| 321 | 0.12 | 1.00 | 2.00 | 17.0-20.0 | 9.0-12.0 | 4x(C) | ||
| 347 | 0.08 | 1.00 | 2.00 | 17.0-20.0 | 9.0-13.0 | 10x(C) | ||
| 316 | 0.08 | 1.00 | 2.00 | 16.0-18.0 | 10.0-14.0 | 2.0-3.0 | ||
| 316L | 0.03 | 1.0 | 2.0 | 16.0-18.0 | 10.0-14.0 | 2.0-3.0 | ||
| 309 | 0.20 | 22.0-24.0 | 12.0-15.0 | |||||
| 310 | 0.25 | 24.0-26.0 | 19.0-22.0 | |||||
Type 300 series are more commonly used in chemical processes for their excellent corrosion resistance. Type 304, or 18/8 as its known for its 18% chromium and 8% nickel content, uses the minimum amount of chromium and nickel to still have its stable austenitic structure. This keeps its price low while still having excellent properties. The carbon content is usually low enough to prevent weld decay making the lack of potential heat treatment not a problem. Type 304L has even less carbon that type 304 (less than 0.03%) and is usually used in areas where carbide precipitation would occur for type 304 (e.g. thicker welded sections). For high temperature use, there is type 321, which is more temperature resistant and is a stabilized version of type 304. Types 309 and 310 can prevent oxidation at high temperatures due to increased chromium content. For even more corrosion resistance, type 316 is the best for its added molybdenum, which can thwart corrosion from dilute sulfuric acid (Towler et al., 2013).
Nickel, Copper, and Monel
Nickel, by itself, has unique inherent advantages to other materials. Unlike stainless steel, nickel is easily workable and not subject to corrosion cracking. This is due nickel's slow rate of oxidation at room temperature. Nickel is typically used in equipment that handle caustic alkalis at temperatures above 70 oC. This is because that is the limit at which carbon steels can operate, thus Nickel acts as a strong replacement (Towler et al., 2013).
Nickel is typically used in processing equipment as it is particularly good at maintaining product purity. These products can range from food to synthetic fibers. It is rare in chemical processes to use pure nickel as making a nickel alloy is a very easy process and can greatly improve certain material properties. Nickel has complete solid solubility with copper, which makes forming alloys with copper simple (Ulrich, 1984).
Copper is a soft metal which is equally easy to work with as Nickel and is found in abundant supply. Its soft nature provides it with good electrical and thermal conductivity. Copper is common in the food industry as well, typically in brewing. Transfer tubes and small-bore pipes often contain copper for its strong heat transfer properties. Copper doesn't react with water but it does slowly react with oxygen in the air. This reaction doesn't produce rust but rather a layer of copper dioxide which protects copper from corrosion. Copper is resistant to caustic alkalis, except ammonia, and many salts and organic acids. Mineral acids are harmful to copper, however (Towler et al., 2013).
Copper can form many alloys. The main ones are brasses (alloyed with zinc) and bronzes (alloyed with tin). They are nearly equally corrosion resistant to metals. Their main use is in valves and other small fittings along with pipes (Towler et al., 2013). As temperature goes down, copper alloys become more ductile and even stronger. An alloy with 70% copper that contains nickel and other strengthening elements is called a "cupronickel" alloy. Cupronickels can easily be fabricated due to the complete solubility between copper and nickel. Cupronickels are strong against seawater corrosion and are resistant to macrofouling and thus are often used in marine applications (e.g. seawater desalination plants, offshore oil platforms, etc). Choosing the right alloy depends on what properties one would like most expressed from thermal conductivity to tensile strength (CDAI).
| Alloy | Density g/cm3 |
Thermal conductivity W/(m·K) |
Thermal expansion µm/(m·K) |
Electrical resistivity µOhm·cm |
Elastic modulus GPa |
Yield strength MPa |
Tensile strength MPa |
|---|---|---|---|---|---|---|---|
| 90-10 | 8.9 | 40 | 17 | 19 | 135 | 105 | 275 |
| 70-30 | 8.95 | 29 | 16 | 34 | 152 | 125 | 360 |
| 66-30-2-2 | 8.86 | 25 | 15.5 | 50 | 156 | 170 | 435 |
Monel is a nickel alloy compromised of mostly nickel (~65%) and copper with other strengthening elements like iron. Monel is stronger than pure nickel and is considered a "single-phase alloy" due to the compatibility of nickel and copper. It is the second most commonly used alloy in chemical plants and is right up there with stainless steel in terms of its widespread uses. It also has specific advantages in certain situations when compared with stainless steels, such as when working with dilute mineral acids and working under reducing conditions. Its only big negative is its price when compared with steel (Towler et al., 2013). Monel is very difficult to machine due to work-hardening which makes its production cost increase relative to its material cost (Monel can be up to 10 times more expensive than nickel and copper). Monel is therefore typically used in situations where it has its inherent advantages and where its cost is outweighed by its benefits. Monel is used in many of the same industries as cupronickel alloys, including marine engineering and oil refinery. However, one must be careful not to put monel near steel in seawater due to the potential of galvanic corrosion (Ulrich, 1984).
Aluminum Alloys and Titanium
Pure aluminum is highly resistant to corrosion, more so than its alloys. It comes from its formation of a thin oxide film, similar to stainless steel. However it lacks mechanical strength which is why pure aluminum is rarely used. The "Duralumin" or "Dural" range of aluminum alloys are most common with small percentages of copper and magnesium (<5%). These alloys are corrosion resistant and strong. They are used in the textile and food industries in places where mild steel may cause contamination (Towler et al., 2013). Aluminum is often appropriate for cryogenic operations (Turton et al., 2012).
Titanium is growing in use in the chemical industry due to its resistance to seawater corrosion and chlorine cracking. It isn't as strong as stainless steel for holding loads but it can withstand more strain which may be of use in liquid oxidation processes. Its thermal conductivity properties make it a good replacement for cupronickel (Towler et al., 2013).
Plastics
Plastics are becoming more commonly used when corrosion is expected. Plastics are also favored because they are inexpensive. However, they have low strength compared to metals (Ulrich, 1984). Plastics can be subdivided into several categories. The first of these is thermoplastic materials, which soften with increasing temperature. PVC falls within this category, and is the most commonly used thermoplastic material in chemical plants. The second category is thermosetting materials, which have a more rigid structure due to cross-linking. Rubber is also often used in linings for tanks and pipes (Towler et al., 2013). Table 4 lists some properties of common plastics.
| Material | Tensile Strength (N/mm^2) | Elastic Modulus (kN/mm^2) | Density (kg/m^3) | Relative Cost |
|---|---|---|---|---|
| PVC | 55 | 3.5 | 1400 | 1.5 |
| Polyethylene (low density) | 12 | 0.2 | 900 | 1.0 |
| Polypropylene | 35 | 1.5 | 900 | 1.5 |
| PTFE | 21 | 1.0 | 2100 | 30.0 |
Inorganic Nonmetals
Inorganic nonmetals include glass, stoneware, brick and cements (Peters et al., 2003). Ceramics are generally stronger than other materials, especially at higher temperatures, they are much more brittle (Ulrich, 1984). Glass is good for corrosion resistance; stoneware is generally corrosive-resistant with more strength, but has poor thermal conductivity (Peters et al., 2003). Ceramic materials possess a cross-linked structure that may be crystalline or partly crystalline and possess a large range of properties across the different types.
Borosilicate glass, composed of silica and boron trioxide, is a very commonly used glass for chemical plants. Also widely known as “Pyrex,” this glass is stronger and can withstand high operating temperatures up to 700 C. In regards to pressure, borosilicate glass cannot withstand high pressures. Therefore, a safety precaution to consider during design is protecting the equipment with an external shielding, and allow for venting to the atmosphere to allow relief for any build up of pressure. Under these constraints, this material is often used for small-scale manufacture of specialty chemicals. It can be used to create pipes and fittings over a range of sizes, and is commonly used to manufacture distillation columns, absorption columns and heat exchangers. Glass is also often used as a lining, because of its relatively chemically inert properties. For example, glass is resistant to acids, salts and other organic materials. It is important to note, however, that glass is not resistant to alkalis and fluorine.
Stoneware is a better quality version of borosilicate glass, as it is stronger but carries similar properties and applications as borosilicate glass. It is often used for packing distillation and absorption columns.
Refractory materials are chemically and physically stable at high temperatures, therefore making them good contestants for manufacturing fired heaters and boilers. They are composed of silica (SiO2) and alumina (Al2O3), and the varied balances of these two compounds strongly affect the performance of the material. Forming a eutectic system at 1545C of 99.45% SiO2, it is important to keep the composition away from this breakdown. A eutectic system describes a homogenous solid mix, and at this point, the system will melt as a whole. Therefore, to ensure that the equipment will not experience distortion and melt, the composition of the refractories must be either above or below 99.45% SiO2. Silica bricks contain >98% SiO2, and are used for general furnace composition. Alumina bricks contain 60% Al2O3, and are used for special furnaces. Fire bricks, composed of 50% SiO2, 40% Al2O3, balance CaO and Fe2O3 is also used for general furnace construction. One property of silica is that it can expand at certain temperatures, and therefore this needs to be taken to account during process design and operation (Towler et al., 2013).
Just like any other material, there are advantages and disadvantages in using glass. One advantage is that glass is resistant to most acids. One notable exception is that it is not resistant to hydrofluoric and hot, concentrated H3PO4. Another advantage is that by using glass piping, the contents of the pipes can be seen and observed if that is necessary or helpful to the process. The main disadvantage of using glass is its brittleness. Glass is also subject to attack by hot alkaline solutions and to damage by thermal shock. Measures can be taken to armor the glass to reduce breakage. This can be done with an epoxy-polyester fiberglass. Utilizing the advantages of glass with the advantages of other materials also makes for great materials of construction. For example, using glassed steel allows for the corrosion resistance from the properties of glass, but also allows for a strength due to the properties of steel. As mentioned earlier, glass linings are also highly utilized in industry. Glass linings are resistant to: all concentrations of HCl up to 120C, dilute concentrations of sulfuric to the boiling point, concentrated sulfuric to 230C, and all concentrations of nitric acid to the boiling point (Perry et al., 2008).
Case Study
Example 1
Example Problem: Chlorobenzene is produced by reacting liquid benzene with gaseous chlorine. The reaction takes place at 328K and 2.4 bar. If both the feeds are at 293K and atmospheric pressure, what are appropriate materials for the inlet piping and the reactor? (Adapted from Chemical Engineering Design (Towler et al., 2013)).
Both benzene and dry chlorine are not corrosive and therefore, carbon steel can be used as the inlet piping. Note that if the gaseous chlorine is actually wet chlorine, it becomes very corrosive to most metals and a plastic should likely be used. While the reactor is at higher pressure than atmospheric pressure, it is well below the maximum allowable stress for all common materials (Towler et al., 2013). However, a side product of the reactor is HCl which is corrosive. Likely, the HCl concentration will not be high enough to corrode the material but this should be investigated further. If concentration is >50% either in the reactor or later in the process, another material such as a plastic should be
Example 2
Corrosion must be taken into account while designing a pressure vessel. Equipment thickness must be able to handle the wear and tear of plant operation. In general, corrosion allowances are about 1.5-5mm thick (Towler et al., 2013).
Example Problem: Consider a packed bed catalytic reactor for the reaction of propylene with hydrogen peroxide to form propylene oxide. The propylene feed rate is 10,000 lb/h at 60C. The reactor is isothermal and operates at 500 psi. The catalyst possesses a surface density of 0.25 sites/nm2, a particle surface area of 800 m2/g, a particle density of 1 g/cm3, a diameter of 250 micrometers, and a void fraction of 0.4. The reactor volume is 1.16 m3, with a diameter of 1 m, and length of 2 m. There is an allowance of 0.5 m on each end of the catalyst bed for internals. What is the estimated wall thickness?
The design pressure of this pressure vessel will be 550 psi, with a design temperature of 88 C. Due to the corrosive media of hydrogen peroxide, stainless steel should be used, and the corrosion allowance is 4 mm. However, hoop stress and longitudinal stress needs to be taken into consideration to determine the necessary thickness of the pipes.
Hoop stress can be calculated by the equation
Pi = internal pressure, D = internal diameter, S = maximum allowable stress (found in literature tables), E = welded joint efficiency (based on type of weld). In this example, hoop stress is 15.3 mm.
Longitudinal stress can be calculated by the equation
Here the longitudinal stress is 7.5 mm
Hoop stress dominates as it is greater than the calculated longitudinal stress thickness, so the hoop stress added on with the corrosion allowance = 15.3 mm + 4.00 mm = 19.3 mm. Therefore, the wall thickness should be 19.3 mm.
Example 3
Example Problem: Chemical Corporation X (CCX) is known for their highly efficient and cost-effective production pathway for producing ethylene. Due to pressures from newer competitors in the market, the research and development team decides to find ways to cut cost . A researcher discovers a new material he names Material Y. It has a density of 16000 kg/m3 s cost of production is $2.00/kg. Its maximum allowable stress is 0.14 N/mm2, which is the same as the stainless steel grade CCX uses for its now aging storage tanks. Would it be worth it for CCX to use this new Material Y in place of purchasing their usual stainless steel grade material for its storage tanks? The stainless steel is being purchased at $3.30/kg currently and its density is 8000 kg/m3.
Without much more information to go by, one can only make initial cost comparisons. This is what the cost rating equation is useful for. Using the equation below (Towler et al., 2013):
Inputing the information from the example results in a cost rating of 188,571 for stainless steel and a cost rating of 228,571 for Material Y. Material Y is roughly 1.2 times as expensive stainless steel when comparing the relative cost ratings despite Material Y's lower price per kilogram. CCX should stick with their current stainless steel grade for their storage tanks.
References
Biegler LT, Grossmann IE, Westerberg AW. Systematic Methods of Chemical Process Design. Upper Saddle River: Prentice Hall; 1997.
Callister W, Rethwisch D. Materials Science and Engineering. Wiley: New York, 2011.
Copper Development Association Inc. Copper.org [Internet]. Physical Properties of Copper Nickel Alloys [2000]. Available from: http://www.copper.org/applications/marine/cuni/properties/physical/
Engineering Archives Website. Stress Strain Diagram. http://www.engineeringarchives.com/les_mom_stressstraindiagram.html.
Perry R, Green D. Perry’s Chemical Engineer’s Handbook. 8th ed. New York: McGraw-Hill; 2008.
Peters MS, Timmerhaus KD. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw Hill; 2003.
Stainless Steel Industry of America. The Stainless Steel Information Center [Internet]. Relationship between hardness and strength [February 2013]. Available from: http://www.ssina.com/overview/history.html
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.
Turton R, Bailie RC, Whiting WB, Shaewitz JA, Bhattacharyya D. Analysis, Synthesis, and Design of Chemical Processes. 4th ed. Upper Saddle River: Prentice-Hall; 2012.
Ulrich, GD. A Guide to Chemical Engineering Process Design and Economics. Wiley: New York, 1984.
VanAken D. Industrial Heating Website [Internet]. Relationship between hardness and strength [updated 2001 Mar 7; cited 2015 Mar 1]. Available from: http://www.industrialheating.com/articles/84495-engineering-concepts-relationship-between-hardness-and-strength?v=preview.