Shale Gas to Ethylene (G2): Difference between revisions
| Line 13: | Line 13: | ||
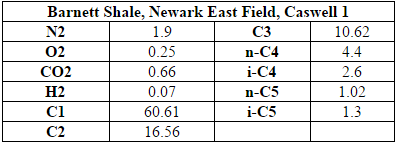
The inlet to the process is raw shale gas that is taken from the Barnett Shale site in Texas (specifically, shale gas extracted from well C1 of the Newark East field). The inlet flow rate of shale is 171,200 kg/hr and the composition of this raw shale gas is shown in Table A.1 of the appendix (American Petroleum Institute, 2011). It is assumed that there is little to no sulfur in the raw shale gas. The plant will be located nearby in Johnson County, Texas. This location is near the extraction site of the Barnett shale gas to avoid large transportation distances and costs but far enough away from the Dallas-Ft. Worth area to ensure adequate land availability and privacy. |
The inlet to the process is raw shale gas that is taken from the Barnett Shale site in Texas (specifically, shale gas extracted from well C1 of the Newark East field). The inlet flow rate of shale is 171,200 kg/hr and the composition of this raw shale gas is shown in Table A.1 of the appendix (American Petroleum Institute, 2011). It is assumed that there is little to no sulfur in the raw shale gas. The plant will be located nearby in Johnson County, Texas. This location is near the extraction site of the Barnett shale gas to avoid large transportation distances and costs but far enough away from the Dallas-Ft. Worth area to ensure adequate land availability and privacy. |
||
While ethylene is the only product chemically produced, hydrogen, methane, propane, and NGLs are recovered in the process and can be sold. To be sold, the following purities must be met or exceeded: 98% pure ethylene, 90% pure hydrogen, 95% pure methane and propane, and 95% purity NGLs (Ethylene Gas Specifications, 2014 and Keller, 2012) Thus, the approach taken in the development of this design is to take raw inlet shale, refine it to separate out methane, ethane, propane and NGLs and then use the ethane to produce ethylene via steam cracking. Further processing and refinement also leads to the recovery of hydrogen (an input added to the hydrogenation reactor and produced in the steam cracking process). |
While ethylene is the only product chemically produced, hydrogen, methane, propane, and NGLs are recovered in the process and can be sold. To be sold, the following purities must be met or exceeded: 98% pure ethylene, 90% pure hydrogen, 95% pure methane and propane, and 95% purity NGLs (Ethylene Gas Specifications, 2014 and Keller, 2012). Thus, the approach taken in the development of this design is to take raw inlet shale, refine it to separate out methane, ethane, propane and NGLs and then use the ethane to produce ethylene via steam cracking. Further processing and refinement also leads to the recovery of hydrogen (an input added to the hydrogenation reactor and produced in the steam cracking process). |
||
=Design Summary= |
=Design Summary= |
||
Revision as of 18:22, 13 March 2015
Executive Summary
This report is part of an ongoing project to design a process to produce ethylene and NGLs from shale gas. The inlet raw shale gas is taken from the Barnett Shale site in Texas with an inlet flow rate of shale is 171,200 kg/hr. The design would be implemented in Johnson County, TX, which is nearby the shale site. The Barnett shale contains about 17% ethane, which we seek to separate from the inlet gas and convert into ethylene via steam cracking. Additionally, because the shale contains significant amounts of other hydrocarbons, natural gas, propane, and NGLs will be recovered from the shale to be sold.
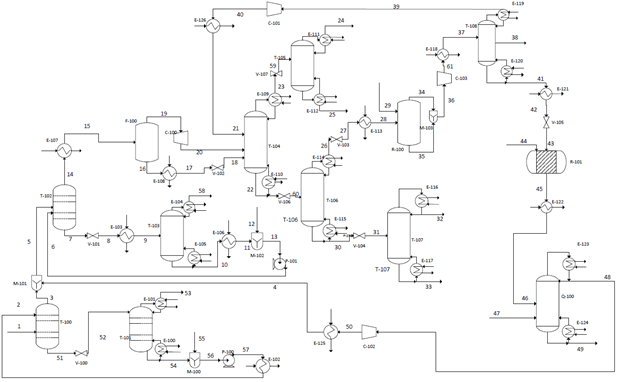
The proposed design consists of six main sections which were modeled in HYSYS: acid gas removal, dehydration, fractionation train, hydrogenation reactor, ethylene splitter, and steam cracking. The acid gas removal section uses Diethylamine (DEA) to remove the CO2 from the raw shale gas. The DEA is fully recovered, thus no make-up stream is required, and the CO2 content is reduced from 0.66 to 0.04 mol%. The purpose of the dehydration section is to remove all of the water from the shale before it is sent through the fractionation train, as water will freeze due to the low temperatures in this section. Triethylene glycol (TEG) is used to absorb water from the gas, reduce the water content from 2.44 to 0.01 mol%. TEG is then partially recovered and recycled. The fractionation train consists of 4 distillation columns. The first is the demethanizer, which yields a methane-rich tops that is sent to a second distillation column to purify methane for sale, and the bottoms is sent to the third column, the deethanizer. The deethanizer ethane-rich tops is sent on for further processing, while the bottoms is sent to the fourth column, the depropanizer, with yields a propane top stream and an NGL bottom stream. The next section is the hydrogenation reactor, into which the tops of the deethanizer is fed to selectively hydrogenate acetylene, which is an impurity from the cracking reactor, into ethylene and ethane, reducing the content of acetylene in the gas from 100 ppm to 4 ppm. Next, they hydrogenated gas proceeds to an ethylene splitter which separates ethylene for sale, methane to recycle, and ethane which proceeds to the final section, the cracking reactor. The cracking reactor operates at about 820C and converts ethane to ethylene, with the cracked gas containing 48% ethylene. The cracked gas is then quenched and recycled for purification.
This discussed design results in the following product streams: 26,600 kg/hr of >98% purity ethylene , 6,300 kg/hr of 94% pure hydrogen, 68,600 kg/hr of 96% pure methane, 30,600 kg/hr of 97% pure propane, and 38,200 kg/hr of NGLs, which is 72% butane and 26% pentane. The total profits from the sale of these products surmounts to about $328 million annually. The annual cost of this design includes the cost of purchasing feeds, offsite organic waste treatment, utilities (determined from Aspen Energy Analyzer), labor, and other miscellaneous costs. This annual cost is $250 million. Additionally, the total capital cost, determined from estimated equipment sizes as well as added contingency for piping and other fixtures, sums to $120 million. From these costs, the design has a 10 year NPV of $547 million, a payback period of 3 years and an internal rate of return (IRR) of 72%. These calculations indicate a profitable design that should be pursued. However, this design and the resulting calculations should be refined with additional researching and alternatives beyond what is presented in this report.
Introduction
This report summarizes a project to design a process to produce ethylene and NGLs from shale gas. The production of ethylene is desirable because ethylene is a valuable product in the production of polymers, chemicals and other consumer products. In this final report, the design and simulation in HYSYS are discussed in detail. In addition, the rationale behind the selection of the specific technology chosen for the design is provided. The objectives when choosing specific technology were to maximize efficiency and conversion while minimizing capital and operating costs of the facility. Finally, an economic analysis was conducted, including capital costs, variable costs and fixed costs analyses with an overall NPV calculation. Additionally, a sensitivity analysis was conducted to determine the effect of certain design tradeoffs and changes in market pricing.
Technical Approach
The inlet to the process is raw shale gas that is taken from the Barnett Shale site in Texas (specifically, shale gas extracted from well C1 of the Newark East field). The inlet flow rate of shale is 171,200 kg/hr and the composition of this raw shale gas is shown in Table A.1 of the appendix (American Petroleum Institute, 2011). It is assumed that there is little to no sulfur in the raw shale gas. The plant will be located nearby in Johnson County, Texas. This location is near the extraction site of the Barnett shale gas to avoid large transportation distances and costs but far enough away from the Dallas-Ft. Worth area to ensure adequate land availability and privacy.
While ethylene is the only product chemically produced, hydrogen, methane, propane, and NGLs are recovered in the process and can be sold. To be sold, the following purities must be met or exceeded: 98% pure ethylene, 90% pure hydrogen, 95% pure methane and propane, and 95% purity NGLs (Ethylene Gas Specifications, 2014 and Keller, 2012). Thus, the approach taken in the development of this design is to take raw inlet shale, refine it to separate out methane, ethane, propane and NGLs and then use the ethane to produce ethylene via steam cracking. Further processing and refinement also leads to the recovery of hydrogen (an input added to the hydrogenation reactor and produced in the steam cracking process).