Design G2
Author: Sean Cabaniss, David Park, Maxim Slivinsky, and Julianne Wagoner (Winter 2014)
Steward: David Chen, Fengqi You
Executive Summary
Biodiesel is a biofuel alternative to petroleum diesel. One of the main pathways of biodiesel production is through transesterification. For each unit of biodiesel converted using this reaction, approximately 10% by weight will be recovered as by-product glycerol. This reaction alone accounted for approximately 65% of total glycerol production in 2011. The growing biodiesel market has created an abundance of inexpensive glycerol, which can be converted into higher value products such as propylene glycol. After conducting a thorough review of the literature, a process was developed based on existing UOP patented technology. This process produces propylene glycol via hydrogenolysis of glycerol. The reaction is carried out at 370 °F and 800 psi, which results in 85% conversion of glycerol with a 91% selectivity to propylene glycol, balance ethylene glycol. The main product is purified to 99.8 wt% to meet USP/EP grade. The main byproduct, ethylene glycol, is sold at 99.9 wt%. The process was simulated in Aspen HYSYS V7.3 to determine material balances and overall energy requirements. The process uses 16,919 tons of crude glycerol a year to produce 9,601 tons of propylene glycol and 759 tons of ethylene glycol year. This requires 823,680 tons of water, 609,840 tons of steam and 229,680 kWh a year. The sizing and cost analysis for each of the individual machines and utilities as well as the overall economic analysis have also been examined. The project is estimated to cost 7.63 $MM in capital and 12.3 $MM annual cost of production. The total project revenue comes out to 25.9 $MM each year. After an economic analysis the process was determined to have a 10 year NPV of 4.11 $MM and 20 year NPV of 7.9 $MM with respective IRR of 30% and 34%. These numbers were calculated using 20% cost of capital, a 34% tax rate and a 10 year MACRS depreciation. The project was deemed to be highly profitable and is recommended to move forward when possible.
Introduction
Various political, economic, and environmental concerns over the past decades have led to a desire to decrease dependence on fossil fuels for energy. One alternative is biofuel, or fuel derived from living organisms. Several countries and organizations have worked to promote the use of biofuels. In the United States, the Energy Independence and Security Act (EISA) of 2007 mandated that the volume of renewable fuels blended into transportation fuels be 36 billion gallons by 2022 (The Energy Independence and Security Act of 2007, 2007). Biodiesel is a biofuel alternative to petroleum diesel. One of the main pathways of biodiesel production is through transesterification. For each unit of biodiesel converted using this reaction, approximately 10% by weight will be recovered as by-product glycerol (Davyprotech.com). This reaction alone accounted for approximately 65% of total glycerol production in 2011 (Transparencymarketresearch.com). The growing biodiesel market has created an abundance of inexpensive glycerol, which can be converted into higher value products such as propylene glycol.
Design Basis
Market Analysis
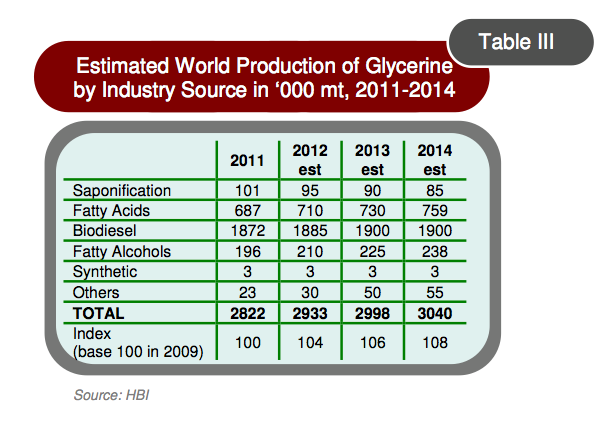
The overabundance of glycerol caused by the growing biodiesel market has driven prices for glycerol to about $200/ton (Alibaba.com; Bozell and Petersen, 2010). As shown in Figure 1, the supply of glycerol will continue to outpace the demand in 2014 at a growth rate of 2.5% per annum (Oleoline.com).
Figure 1. Global glycerine production in various industrial sectors.
The production grades of glycerol are crude, technical grade, and USP (United States Pharmaceutical) grade. Crude glycerol comes from production of biodiesel and contains 40-88% glycerol with significant amounts of salt, water, soaps, and methanol. Technical grade glycerol is a refined product with a minimum 98% glycerol content and no salt, soaps, methanol, or other contaminants. USP grade glycerol is a pharmaceutical grade for use in the food, pharmaceutical, and cosmetics industries (Srsbiodiesel.com).
Commercial sources of glycerol other than biodiesel production include fatty acids, fatty alcohols and from the soap industry via the saponification process (Bozell and Petersen, 2010). Glycerol is recognized as safe for animals and humans and environmentally benign, with no significant environmental regulations.
Propylene glycol is conventionally produced using propylene oxide. It is, therefore, sensitive to the price and availability of petroleum and associated products (Davyprotech.com). For this reason, propylene glycol is relatively expensive at around $2500/ton (Interview with Dow Chemical). Supply of propylene glycol struggles to keep up with an increasing annual global demand currently at 1.8m tons (Prweb.com). The ability to isolate propylene glycol production from petroleum by using inexpensive glycerol as a feedstock would be hugely advantageous.
Propylene glycol is used in several applications, including the food, pharmaceutical, and cosmetics industries, as well as in liquid detergents, functional fluids, and unsaturated polyesters (Nizamoff). The two grades of propylene glycol are industrial (99.5% purity) and USP/EP (99.8% purity) (Oleoline.com). Like glycerol, propylene glycol is recognized as safe for animals and humans. Because propylene glycol is biodegradable, it is not considered harmful to the environment and, thus, there are no significant environmental regulations.
Process Technology
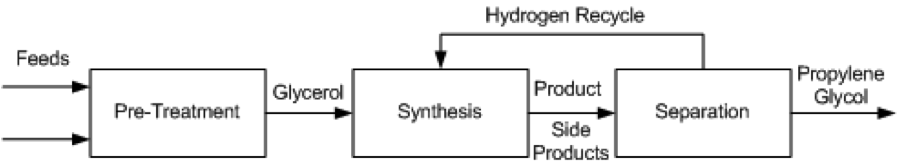
Several different processes have been proposed for the conversion of glycerol to propylene glycol. These include UOP (Bricker and Leonard, 2012), Davy Process Technology (Tuck, 2012), GTC Technology (Ding et al., 2013), the Lanzhou Institute process (Cui et al., 2009), the Petroleo Brasileiro (Rabello et al., 2011) process, and ADM (Bloom, 2011). These methods all employ catalytic hydrogenolysis and proceed using the same general pathway, show in Figure 2.
Figure 2. Block Flow Diagram of process alternatives
Pre-Treatment
- The production of propylene glycol from glycerol requires technical grade glycerol, which means a crude glycerol feed must undergo pre-treatment before entering the reactor. For different grades of glycerol the specific process will change, but it will generally be necessary for feeds to be purified, mixed, and heated before high purity glycerol is sent to the synthesis stage. In the GTC process, glycerol, hydrogen and methanol are mixed and heated to anywhere from 150 °C to 240 °C, at pressures between 20 and 80 atm. The preferred composition of the mixture assumes an already pure glycerol feed to be mixed, so any glycerol purchased at lower purities must be distilled to purity before entering the mixer and heater. The Lanzhou and Petroleo Brasileiro processes describe vacuum filtration and distillation of crude glycerol to remove impurities such as sodium, chloride, sulfur and phosphorous salts, fatty acids, phospholipids, glycerides, soaps and biodiesel residues. Any of these impurities can kill the catalyst used downstream. The treated glycerol purity is between 90 – 100%.
Synthesis
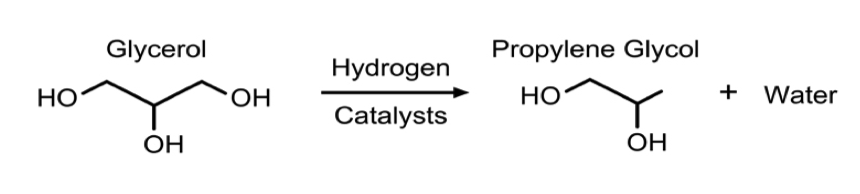
- In all process technologies considered, the basis of the synthesis is hydrogenolysis of glycerol via packed bed reactors with some form of a catalyst, usually copper based. This reaction is shown in Figure 3. In some cases, the process allows for more reactors to be used in series to achieve a higher conversion. Much of the variation in the processes being examined is based on different operating conditions and the desired purity of the product, propylene glycol.
Separation
- A series of separations is used to separate by-products from propylene glycol. Three step distillations are common; some procedures allow for additional steps, which can change the purity of the product. Common byproducts that need to be separated are methanol, acetol, water, and various other minor alcohol solutions.
Site Conditions and Capacity
In the United States, the EPA biofuel mandate for 2014 will be reduced from 18.15 billion gallons to 15-15.52 billion gallons (United States Environmental Protection Agency), so the production of biodiesel will decrease, decreasing the supply of crude glycerol in the United States. In South America, Argentina and Brazil are the largest producers of biodiesel, with production in Brazil growing at the fastest rate. It is estimated that 25-30% of Brazilian glycerol production went to drain in 2010 and 2011, indicating a large supply of inexpensive feedstock (Oleoline.com). Building a facility in Salvador da Bahia, Brazil not only enables access to this supply of inexpensive glycerol, but also provides access to a port city and thus allows export of propylene glycol to high demand markets such as China and the U.S. Additional benefits of building in Brazil include the lower corporate tax rate at 34% compared to 40% in the United States (Kpmg.com) and the temperate climate with an almost constant average temperature of 80 °F (Wmo.int). Dow Chemical currently operates a conventional propylene glycol facility near Salvador, indicating a potentially strong market in the area (Dow.com). The capacity selected for this project is 10,000 ton/year. Current plants using comparable technology, such as ADM and Oleon operate at 100,000- and 200,000-tons, respectively (Icis.com, a). The plant capacity is therefore relatively small, which leaves room for increased production.
Process Model Basis and Assumptions
Reactor
The process is based on the design outlined by UOP (Bricker and Leonard, 2012). The reaction is catalytic hydrogenolysis of glycerol to propylene glycol over a Co/Pd/Re catalyst consisting of 2.5 wt% Co, 0.4 wt% Pd, and 2.4 wt% Re on NORIT ROX 0.8. The catalyst was reduced at 320 °C in the presence of only H2 prior to use in the reactor. The reaction is carried out at 225.6 °C and 5516 kPa with a 1.17 LHSV. The feed enters the reactor at a Hydrogen to glycerol feed ratio of 2.5:1 and at a pH of 12. At these reactor conditions glycerol conversion and selectivities toward propylene glycol and ethylene glycol are 85%, 91%, and 9%, respectively. The upper bound for reactor methanol concentration was set at 7 wt% to maintain catalyst performance according to specifications outlined by UOP (Bricker and Leonard, 2012).
Feedstocks and Products
The reactor feed glycerol (including pre-treated and recycled glycerol) is at 23.16 °C and 5516 kPa and has a composition of 37.77 wt% glycerol, 54.42 wt% water, .77 wt% NaOH, 3.36 wt% sodium sulfate, 3.63 wt% methanol, and .04 wt% acetic acid (Bricker and Leonard, 2012). Hydrogen gas is purchased at 187.8 °C and 5516 kPa. Our main product, propylene glycol, can be sold at industrial grade purity of 99.5 wt% or USP grade purity of 99.8 wt% (Dow.com). One of our byproducts, ethylene glycol, can be sold at a variety of grades, including Polyester grade (99.9 wt%) and Industrial grade (99.1 wt%) (Meglobal.biz).
Process Overview
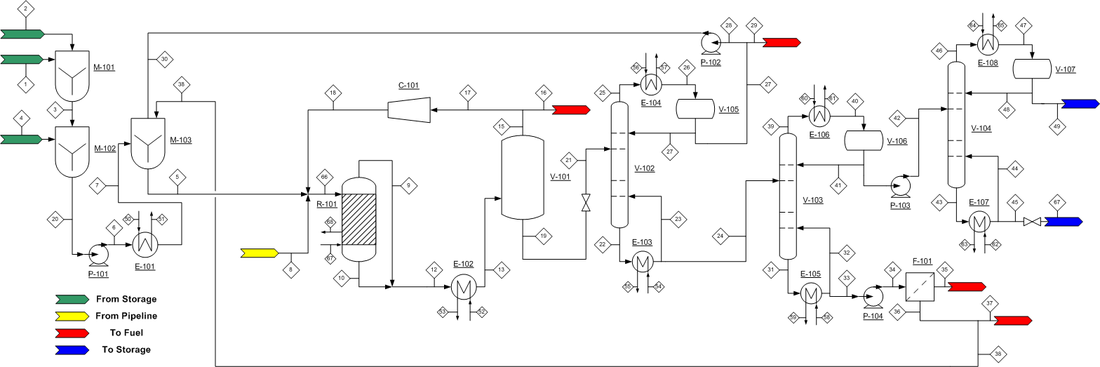
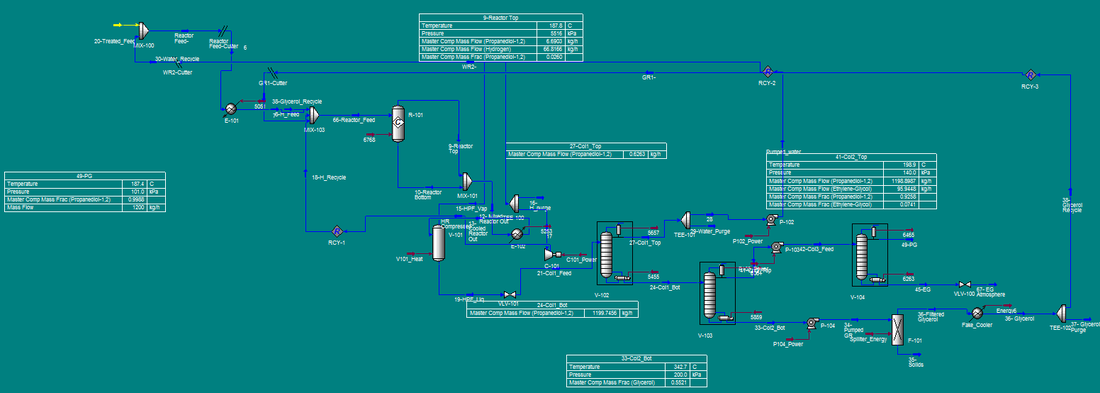
The process flow diagram (PFD) can be found in Figure 4. Incoming glycerol is a byproduct of biodiesel production, usually 40 to 85% glycerol, so it contains fatty acids that must be removed before contacting the fixed-bed reactor catalyst. M-101 mixes the incoming feed with sulfuric acid to remove the fatty acids and produce acidulated glycerol. Acidulated glycerol can contain some amount of methanol, sodium, potassium, sulfur, iron, nickel, chloride or trace impurities. The presence of such impurities in small enough amounts will not negatively affect the production of propylene glycol. The best way to ensure the glycerol mixture will be usable is to ensure that methanol content is <1.5% by weight. The acidulated glycerol is then moved to mixer M-102, where it is contacted with 1.77 wt% aqueous sodium hydroxide. This mixer will increase pH to ~12; a basic glycerol solution will have a much higher selectivity towards propylene. The pH corrected glycerol stream is then heated to 148.9 °C and mixed with water and glycerol recycle streams in M-103. The outgoing glycerol mixture is then mixed with compressed hydrogen gas in a 2.5:1 hydrogen to glycerol mole ratio. The hydrogen comes from an external gas feed. The resulting liquid/gas mixture is sent to the fixed-bed reactor R-101.
Figure 4. Process Flow Diagram for the production of propylene glycol.
Hydrogenolysis of glycerol to propylene glycol is carried out in R-101 at 187.8 °C and 5516 kPa. Due to the exothermic nature of the reaction, it is necessary to provide a quench gas stream. In this case, the recycled hydrogen comes in at 72.8 °C, which maintains the reactor temperature at 187.8 °C. The catalyst utilized is a Pd/Co/Re on NORIT ROX 0.8, which provides an 85% conversion of glycerol, with a 91% selectivity to propylene glycol at the given operating conditions. The reactor effluent contains propylene glycol, unreacted glycerol and other byproducts and hydrogen gas. The effluent is sent to V-101, a flash evaporator, where the hydrogen gas is removed from the stream and split into two directions: to be sent off as waste and to be recycled. The waste stream is useful to remove any unwanted gasses that may accumulate over repeated reaction cycles. The resulting propylene glycol mixture is then sent to V-102 for separation and purification.
V-102, a fractionation tower, removes water and C2 alcohols from the propylene glycol reactor effluent. The overhead stream, containing 96 wt% water and balance C2 alcohols, is recycled. The bottoms of V-102 contain water-free propylene glycol, which is then sent to V-103, another fractionation tower which will separate the desired product from the unreacted glycerol and other byproducts. The overhead stream contains 92.6 wt% propylene glycol. The bottoms stream contains unreacted glycerol, ethylene glycol, sodium salts and other impurities. This is sent to F-101, a solid/liquid filter that will remove the solid salt impurities for disposal. The resulting purified liquid stream can be recycled to the beginning of the process and mixed with incoming feed in M-103.
The overheads of V-103 are sent to V-104, which will separate propylene glycol from ethylene glycol. The resultant overheads are 99.8 wt% propylene glycol, which is sent to a storage tank. Additionally, the bottoms are 99.9 wt% ethylene glycol, which is also stored in a tank.
Process Simulation
The process is modeled in Aspen HYSYS V7.3 using the non-random two-liquid (NRTL) model as the fluid package, the results of which are shown in Figure 5.
Figure 5. HYSYS simulation for the production of propylene glycol.
Optimization
Simple distillation columns in HYSYS were used to find initial estimates for tray numbers, reflux ratios, and optimal feed stage location. Once complex columns were simulated, these specifications were further optimized. Liquid returned to columns via reflux is cooler than up-flowing vapors. Heat transfer between the two components improves the efficacy of the distillation tower, reducing the number of trays needed. However, if a column is operated in total reflux, no product will ever be collected. The price of each column, utilities costs, product yields were optimized by testing several combinations of reflux ratios and tray numbers. The temperature of the inlet stream and component fractions should be similar to the tray the feed enters on. This knowledge was used to optimize the feed tray numbers for each distillation column, decreasing the number of trays needed, the cost of utilities, and increasing the product purity.
Reactor Cost was optimized using Solver in Microsoft Excel 2010. The cost accounted for the pressure drop across the reactor (Ergun equation), minimum volume necessary to meet target LHSV, and design specifications for pressure vessels including wall thickness and diameter, and minimum heat transfer specifications such as area, jacket spacing, jacket type, and heat transfer fluid type. Also, several materials were evaluated, including SS304 and SS407, to find the lowest overall cost.
Waste Streams
The water purge is a dilute aqueous waste stream and will be treated in a wastewater facility at a cost of $1.5/t. The hydrogen and glycerol purge can be used as heating fuels due to their high heating values. This will offset waste treatment costs as well as fuel costs. If the price of heating fuel is taken to be $4.50/GJ (Interview with Dave Wegerer), this results in savings of $638.10/t H2 and $68/t Glycerol purge. The solid waste, Na2SO4, can be sold at around $100/t (Kostick).
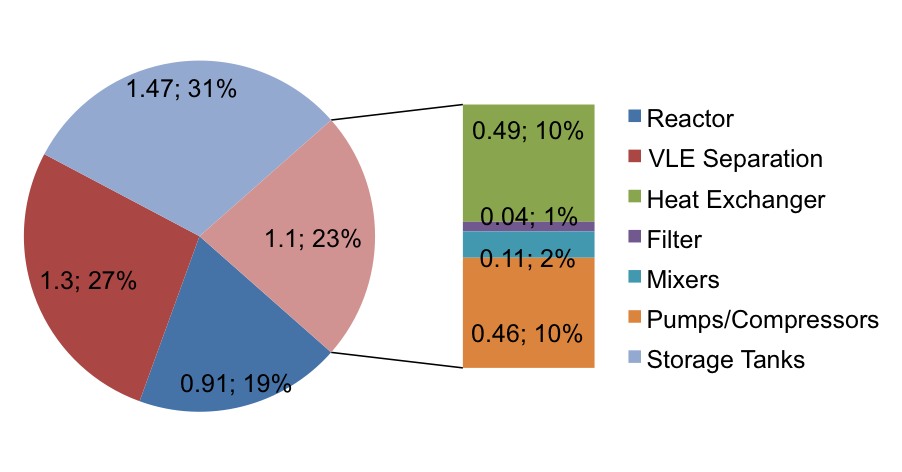
Equipment Costs
Figure 6 below shows the approximated costs of each of the pieces of equipment calculated using Aspen Economic Evaluator v7.3.1. The major components running through the equipment are not corrosive, except basic water. In addition most of the vessels are under fairly standard temperatures and pressures. The key exception is the jacketed reactor, which is subject to extreme conditions. The selection of SS407 allowed for a cheaper reactor as compared to SS304 due to the higher tensile strength. The total ISBL equipment cost is 4.8 $MM in 2010 Gulf Coast USD. The NF cost index is 2250 in 2010 and will conservatively be 2050 in 2014 (Towler and Sinnott, 2013), which adjusts project cost to 5.33 $MM in 2014 Gulf Coast USD. The 2003 location factor for Brazil is 1.14 (Towler and Sinnott, 2013), and the exchange rate in 2003 was 1 Real = $.3402 (Oanda.com). The average rate for the past 3 months has been 1 Real = $.427 (Bloomberg.com, a). The adjusted capital cost for Brazil in 2014 is therefore 7.63 $MM. Since the project is large-volume chemical on a new site, OSBL is taken as 40% of ISBL, or 3.05 $MM. Engineering and contingency costs are taken as 10 and 15%, respectively, of combined ISBL and OSBL costs.
Figure 6. Equipment Cost Breakdown
Prices
The price of feedstocks crude glycerol and hydrogen are $200/t (Alibaba.com; Bozell and Petersen, 2010) and $1100/t (Icis.com, b). The price of products propylene glycol and ethylene glycol are $2557/t (Interview with Dow Chemical) and $1400/t (Meglobal.biz). The price of consumables NaOH and H2SO4 are $635/t (Icis.com, c) and $80/t (Icis.com, d). The catalyst must be replaced every 2 years, at a cost of 5.13 $MM (Basf.com; Lme.com; Sigmaaldrich.com). The price of electricity has been fluctuating recently due to lack of rainfall, and is taken as 0.202 $/kWh (Bloomberg.com, b). Utilities prices for high pressure steam, medium pressure steam, and cooling water are $14.3/t, $12/t, and $.024/t, respectively (Towler and Sinnott, 2013).
Fixed Operating Costs
Based on the plant size, three shift positions with 4.8 operators per shift will comprise the operating labor. A salary of $35,000 is a reasonable estimate of operator wages in Brazil. Supervision is taken as 25% of operating labor, and direct overhead is 45% of labor and supervision. Maintenance is taken as 3% of ISBL Cost, and plant overhead is 65% of labor and maintenance costs. Property and local tax and insurance are both typically 1% of ISBL plus OSBL Cost. Repayment of debt associated with fixed investment is accounted for in the weighted average cost of capital so 0% is taken as fixed cost of production. However, working capital will be funded entirely by debt, so 5% interest of working capital is taken as interest on debt financing.
The plant is scheduled to be constructed over two years, with 40% of capital expenditure being accounted for in year 1. The plan will operate at 70% capacity in year 3 and 100% in the subsequent years. Cost of equity is taken to be 30% based on chemical industry companies (Towler and Sinnott, 2013), adjusting for increased risk in South American ventures. The debt ratio is taken to be 0.4 which allows this project to be financed by corporate bonds that are rated A and above, with a debt cost of capital of 5%. The resulting weighted average cost of capital is therefore 20%. The project will be depreciated using MACRS 10 year depreciation (Icis.com, e) which allows larger tax savings in the near-term, resulting in higher project NPV. The corporate tax rate in Brazil is 34% (Kpmg.com). Working capital is calculated as seven weeks Cash Cost of Production (CCOP) minus two weeks feed plus 1% of Fixed Capital Cost (Towler and Sinnott, 2013).
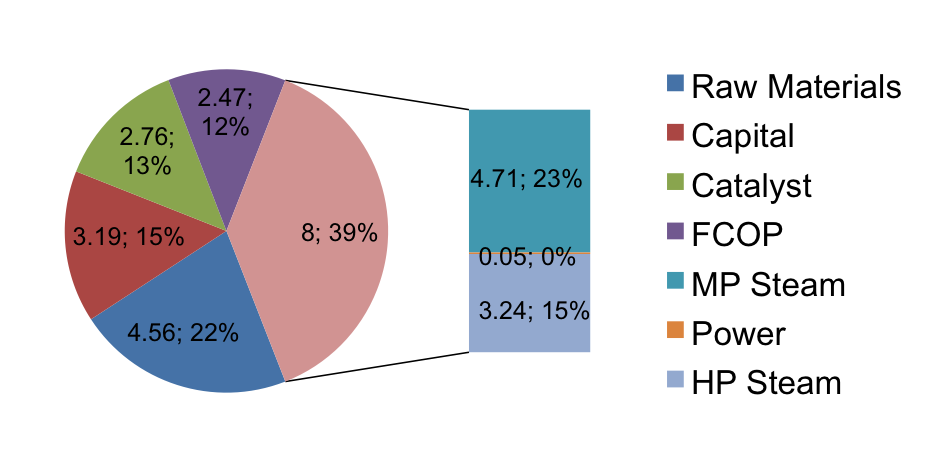
Utilities and Pinch Analysis
The total cost of utilities was found using the energy outputs from HYSYS and known costs of natural gas, water, and electricity in Brazil from commodity indices and surveys from the Brazilian government. The results are presented visually in Figure 7 below. The total utility bill comes to $2,424,000 per year. $1,110,000 from heating gas required to create steam for heating in the process, $1,304,000 in water for both steam generation and cooling water, and approximately $10,000 for electricity to power the pumps and any local offices or break rooms. One important note to consider is that the price of gas in Brazil has risen 40% in the past three months. Continuing fluctuations in energy prices could greatly affect these estimates from year to year.
In order to determine the annual cost of utilities, it was necessary to carry out some heat exchanger design calculations and estimations. After surveying the energy requirement of each exchanger, it was determined that cooling water and steam will be the simplest heat transfer fluids to use, due to the relatively small heat requirements and change in temperature of each process stream. In the case of the three reboilers and three condensers, which are designed with the distillation columns, it was only necessary to find a mass flow rate of steam and water respectively. For the cooling water, once the mass flow rate was calculated, this was sufficient to price. For steam, in addition to purchasing the required mass of water, it was necessary to determine the heat required to raise the steam to the required temperatures. For the one cooler and one heater, we also utilized water and steam, and more thorough design was developed in order to accurately price the two exchangers and to help make a pinch analysis viable.
Figure 7. Utilities Breakdown
Economic Analysis
Gross profits are 8.1 $MM from year 4 onward and the project has a simple payback period of 2.6 years. The project Net Present Value (NPV) for 10 and 15 years is 4.1 $MM and 6.8 $MM. The expected return on this project (10 year IRR) is 30.3%, indicating this project is highly profitable and can be scaled up for higher NPV. Accelerating the project schedule to complete the plant in less than 2 years will also greatly increase the NPV.
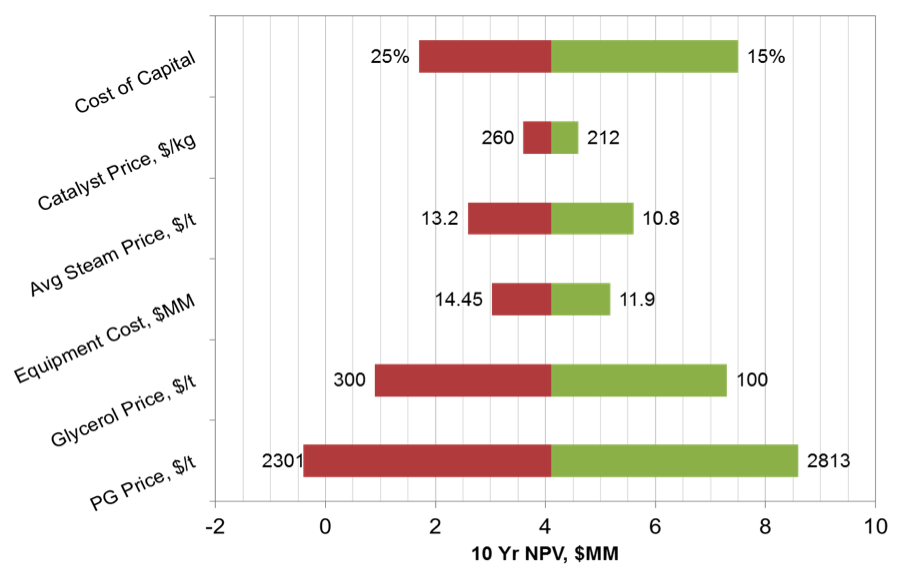
Sensitivity Analysis
A sensitivity analaysis was carried out for a variety of process parameters. For catalyst, PG, HP and MP Steam prices, best- and worst-case were taken as +/- 10% of the base price. The project NPV is most sensitive to the price of Propylene Glycol and Glycerol, which is expected as these are the main product and feedstock. The NPV is also highly sensitive to the cost of capital. The results are presented below, in Figure 8.
Figure 8. Sensitivity Analysis
Conclusion
Our economic analysis has proven that the project as it currently stands is highly profitable and will bring in positive cash flow within three years, although there are some existing environmental and safety concerns. Our current plans involve burning an effluent stream in which the key components are hydrogen, ethylene glycol and some fatty acids. In this case an analysis will need to be done in order to determine the extent of the damage to the local air and if a purification step is necessary. The main safety concerns involve the acid streams and the reactor itself. Operators will needs to be thoroughly educated on acid burn precaution and treatment procedures due to the acidic requirements of the streams. The reactor runs at very high pressures and given the exothermic nature of the reaction appropriate steps will need to be taken in order to ensure that runaway reactions can be safely dealt with and pressure relief systems will be put in place. Our economic analysis has proven that the project as it currently stands is highly profitable and will bring in positive cash flow within three years. However, there is definite room for expansion in the design; our low NPV values and high IRR values indicate the ability to leverage economies of scale and dramatically expand our profit margins. As it stands, we recommend maximizing the NPV of the project with full scale optimization. This entails the addition of parallel reaction trains and the inclusion of a heat exchange network to fully maximize our profit margins. A plant layout should be developed along with the inclusion of automated control schemes to better optimize the process operation. The project currently holds great economic potential and with some more detailed engineering, could provide a very high return for our shareholders.
References
Alibaba.com [Internet]. Hangzhou: Alibaba.com; c1999-2015 [cited 2015 Feb 28]. Available from: http://www.alibaba.com/trade/search?fsb=y&IndexArea=product_en&CatId=&SearchText=glycerol.
Basf.com. Engelhard Industrial Bullion (EIB) Prices [Internet]. Ludwigshafen: BASF Corporation; c2015 [cited 2015 Feb 26]. Available from: http://apps.catalysts.basf.com/apps/eibprices/mp/.
Bloom PD, inventor; Archer Daniels Midland Company, assignee. Hydrogenolysis of Glycerol and Products Produced Therefrom. United States patent WO2008051540 A2. 2011 Apr 19.
Bloomberg.com. BRAZIL REAL-US DOLLAR Exchange Rate [Internet]. New York: Bloomberg L.P.; c2015a [cited 2015 Feb 26]. Available from: http://www.bloomberg.com/quote/BRLUSD:CUR.
Bloomberg.com. Brazilian Power Price Surges to Record Amid Dry Spell [Internet]. New York: Bloomberg L.P.; c2015b [cited 2015 Feb 26]. Available from: http://www.bloomberg.com/news/2014-01-31/brazilian-power-price-surges-to-record-amid-dry-spell.html.
Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010 Apr;12:539-54.
Bricker ML, Leonard LE, inventors; UOP LLC, assignee. Methods for Converting Glycerol to Propanol. United States patent 08101807 B2. 2012 Jul 24.
Cui F, Chen J, Xia C, Kang H, inventors; Lanzhou Institute of Chemical Physics, Chinese Academy of Science, assignee. Method for Producing 1,2-Propylene Glycol using Bio-based Glycerol. United States patent 7586016 B2. 2009 Sep 8.
Davyprotech.com. Licensed Processes Propylene Glycol [Internet]. Johnson Matthey Davy Technologies Limited 2014 [cited 2015 Feb 28]. Available from: http://www.davyprotech.com/what-we-do/licensed-processes-and-core-technologies/licensed-processes/propylene-glycol/specification/.
Ding Z, Chiu J, Jin W, inventors; GTC Technology US LLC, assignee. Process for Converting Glycerin into Propylene Glycol. United States patent 08394999 B2. 2013 Mar 12.
Dow.com. Product Safety Assessment [Internet]. Midland: The Dow Chemical Company; c1995-2015a [cited 2015 Feb 26]. Available from: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_091a/0901b8038091a41a.pdf?filepath=productsafety/pdfs/noreg/233-00490.pdf&fromPage=GetDoc.
Dow.com. Products [Internet]. Midland: The Dow Chemical Company; c1995-2015b [cited 2015 Feb 26]. Available from: http://www.dow.com/propyleneglycol/products/.
Icis.com. Oleochemicals: Oleon enters glycerin-based propylene glycol [Internet]. Surrey: Reed Business Information Limited; c2015a [cited 2015 Feb 26]. Available from: http://www.icis.com/resources/news/2012/07/16/9577645/oleochemicals-oleon-enters-glycerin-based-propylene-glycol/.
Icis.com. Chemical Profile Hydrogen [Internet]. Surrey: Reed Business Information Limited; c2015b [cited 2015 Feb 26]. Available from: http://www.icis.com/resources/news/2005/12/08/190713/chemical-profile-hydrogen/.
Icis.com. Caustic Soda Latin America [Internet]. Surrey: Reed Business Information Limited; c2015c [cited 2015 Feb 26]. Available from: http://www.icis.com/chemicals/caustic-soda/latin-america/?tab=tbc-tab2.
Icis.com. Indicative Chemical Prices [Internet]. Surrey: Reed Business Information Limited; c2015d [cited 2015 Feb 26]. Available from: http://www.icis.com/chemicals/channel-info-chemicals-a-z/.
Icis.com. Figure depreciation under MACRS [Internet]. Surrey: Reed Business Information Limited; c2015e [cited 2015 Feb 26]. Available from: http://www.irs.gov/publications/p946/ch04.html.
Interview with Dave Wegerer on February 25, 2014.
Interview with Dow Chemical on February 13, 2014.
Kostick DS. Sodium Sulfate [Internet]. Reston: United States Geological Survey [cited 2015 Feb 26]. Available from: http://minerals.usgs.gov/minerals/pubs/commodity/sodium_sulfate/620496.pdf.
Kpmg.com. Corporate Tax Rates Table [Internet]. Amsterdam: KPMG International Cooperative; c2015 [cited 2015 Feb 28]. Available from: http://www.kpmg.com/global/en/services/tax/tax-tools-and-resources/pages/corporate-tax-rates-table.aspx.
Lme.com. LME Cobalt [Internet]. London: The London Metal Exchange Limited; c2015 [cited 2015 Feb 26]. Available from: https://www.lme.com/en-gb/metals/minor-metals/cobalt/.
Meglobal.biz. MEG Sales Specifications [Internet]. Washington, D.C.: MEGlobal [cited 2015 Feb 26]. Available from: http://www.meglobal.biz/monoethylene-glycol/sales-specs.
Nizamoff AJ. Green Glycols and Polyols [Internet]. Wheaton: Nexant Inc.; c2011- [updated 2010 Dec; cited 2015 Feb 28]. Available from: http://thinking.nexant.com/sites/default/files/report/field_attachment_abstract/201012/0910S8_abs.pdf.
Oanda.com. Historical Exchange Rates [Internet]. Toronto: OANDA Corporation; c1996-2015 [cited 2015 Feb 26]. Available from: http://www.oanda.com/currency/historical-rates/.
Oleoline.com. Glycerine Market Report [Internet]. Montmorency: HB International SAS; 2012.
Prweb.com. China to Lead PG Market Through 2017, According to Merchant Research & Consulting Ltd Study Available at MarketPublishers.com [Internet]. London: Vocus PRW Holdings, LLC.; c1997-2015 [cited 2015 Feb 28]. Available from: http://www.prweb.com/releases/2013/8/prweb11057161.htm.
Rabello CRK, et al., inventors; Petroleo Brasileiro S.A. Petrobras, assignee. Production of Propylene Glycol from Glycerine. United States patent 20110295044 A1. 2011 Dec 1.
Sigmaaldrich.com. Alternatives for product 39988 Activated Charcoal Norit (FLUKA) [Internet]. St. Louis: Sigma-Aldrich Co. LLC.; c2015 [cited 2015 Feb 26]. Available from: http://www.sigmaaldrich.com/catalog/product/fluka/39988?lang=en®ion=US&fromUrlLabel=product%20details.?lang=en®ion=US
Srsbiodiesel.com. Glycerin Specifications [Internet]. Temecula: SRS International; c2013- [cited 2015 Feb 28]. Available from: http://www.srsbiodiesel.com/technologies/glycerin-purification/glycerin-specifications/.
The Energy Independence and Security Act of 2007: One Hundred Tenth Congress of the United States of America, Pub. L. No. 110-40 (Dec 19, 2007).
Transparencymarketresearch.com. Glycerol Market By Source (Biodiesel, Fatty Acids & Fatty Alcohols), By Applications (Personal Care, Alkyd Resins, Polyether Polyols, Others), Downstream Opportunities (Propylene Glycol, Epichlorohydrin, 1, 3 Propanediol And Others) - Global Industry Analysis, Size, Share, Trends, Growth And Forecast 2012 - 2018 [Internet]. Transparency Market Research. 2013 March [cited 2015 Feb 28]. Available from: http://www.transparencymarketresearch.com/glycerol.market.html.
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.
Tuck MWM, inventor; Davy Process Technology Limited, assignee. Process for the hydrogenation of glycerol to propylene glycol. United States patent 08227646 B2. 2012 Jul 24.
United States Environmental Protection Agency: Office of Transportation and Air Quality. EPA Proposes 2014 Renewable Fuel Standards, 2015 Biomass-Based Diesel Volume [Internet]. [cited 2015 Feb 28]. Available from: http://www.epa.gov/otaq/fuels/renewablefuels/documents/420f13048.pdf.
Wmo.int. Salvador [Internet]. WMO; c2014 [cited 2015 Feb 28]. Available from: http://worldweather.wmo.int/en/city.html?cityId=1081.