Design 2
Executive Summary
Biodiesel is a biofuel alternative to petroleum diesel. One of the main pathways of biodiesel production is through transesterification. For each unit of biodiesel converted using this reaction, approximately 10% by weight will be recovered as by-product glycerol. This reaction alone accounted for approximately 65% of total glycerol production in 2011. The growing biodiesel market has created an abundance of inexpensive glycerol, which can be converted into higher value products such as propylene glycol. After conducting a thorough review of the literature, a process was developed based on existing UOP patented technology. This process produces propylene glycol via hydrogenolysis of glycerol. The reaction is carried out at 370 °F and 800 psi, which results in 85% conversion of glycerol with a 91% selectivity to propylene glycol, balance ethylene glycol. The main product is purified to 99.8 wt% to meet USP/EP grade. The main byproduct, ethylene glycol, is sold at 99.9 wt%. The process was simulated in Aspen HYSYS V7.3 to determine material balances and overall energy requirements. The process uses 16,919 tons of crude glycerol a year to produce 9,601 tons of propylene glycol and 759 tons of ethylene glycol year. This requires 823,680 tons of water, 609,840 tons of steam and 229,680 kWh a year. The sizing and cost analysis for each of the individual machines and utilities as well as the overall economic analysis have also been examined. The project is estimated to cost 7.63 $MM in capital and 12.3 $MM annual cost of production. The total project revenue comes out to 25.9 $MM each year. After an economic analysis the process was determined to have a 10 year NPV of 4.11 $MM and 20 year NPV of 7.9 $MM with respective IRR of 30% and 34%. These numbers were calculated using 20% cost of capital, a 34% tax rate and a 10 year MACRS depreciation. The project was deemed to be highly profitable and is recommended to move forward when possible.
Introduction
Various political, economic, and environmental concerns over the past decades have led to a desire to decrease dependence on fossil fuels for energy. One alternative is biofuel, or fuel derived from living organisms. Several countries and organizations have worked to promote the use of biofuels. In the United States, the Energy Independence and Security Act (EISA) of 2007 mandated that the volume of renewable fuels blended into transportation fuels be 36 billion gallons by 2022 [1]. Biodiesel is a biofuel alternative to petroleum diesel. One of the main pathways of biodiesel production is through transesterification. For each unit of biodiesel converted using this reaction, approximately 10% by weight will be recovered as by-product glycerol [2]. This reaction alone accounted for approximately 65% of total glycerol production in 2011 [3]. The growing biodiesel market has created an abundance of inexpensive glycerol, which can be converted into higher value products such as propylene glycol.
Design Basis
Market Analysis
The overabundance of glycerol caused by the growing biodiesel market has driven prices for glycerol to about $200/ton [4, 5]. As shown in Appendix 7, the supply of glycerol will continue to outpace the demand in 2014 at a growth rate of 2.5% per annum [6].
The production grades of glycerol are crude, technical grade, and USP (United States Pharmaceutical) grade. Crude glycerol comes from production of biodiesel and contains 40-88% glycerol with significant amounts of salt, water, soaps, and methanol. Technical grade glycerol is a refined product with a minimum 98% glycerol content and no salt, soaps, methanol, or other contaminants. USP grade glycerol is a pharmaceutical grade for use in the food, pharmaceutical, and cosmetics industries [7].
Commercial sources of glycerol other than biodiesel production include fatty acids, fatty alcohols and from the soap industry via the saponification process [5]. Glycerol is recognized as safe for animals and humans and environmentally benign, with no significant environmental regulations. The material safety data sheet (MSDS) for glycerol is provided in Appendix 1.
Propylene glycol is conventionally produced using propylene oxide. It is, therefore, sensitive to the price and availability of petroleum and associated products [2]. For this reason, propylene glycol is relatively expensive at around $2500/ton [8]. Supply of propylene glycol struggles to keep up with an increasing annual global demand currently at 1.8m tons [9]. The ability to isolate propylene glycol production from petroleum by using inexpensive glycerol as a feedstock would be hugely advantageous.
Propylene glycol is used in several applications, including the food, pharmaceutical, and cosmetics industries, as well as in liquid detergents, functional fluids, and unsaturated polyesters [10]. The two grades of propylene glycol are industrial (99.5% purity) and USP/EP (99.8% purity) [6]. Like glycerol, propylene glycol is recognized as safe for animals and humans. Because propylene glycol is biodegradable, it is not considered harmful to the environment and, thus, there are no significant environmental regulations. The MSDS for propylene glycol can be found in Appendix 2.
Process Technology
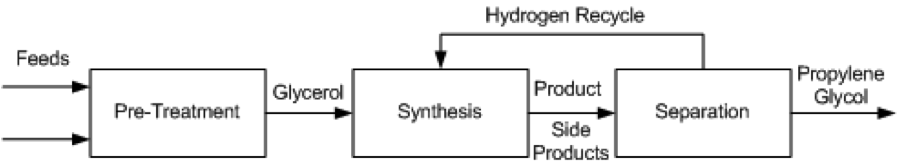
Several different processes have been proposed for the conversion of glycerol to propylene glycol. These include UOP [11], Davy Process Technology [13], GTC Technology [12], the Lanzhou Institute process [14], the Petroleo Brasileiro [15] process, and ADM [16]. These methods all employ catalytic hydrogenolysis and proceed using the same general pathway, show in Figure 1.
Figure 1. Block Flow Diagram of process alternatives
Pre-Treatment
- The production of propylene glycol from glycerol requires technical grade glycerol, which means a crude glycerol feed must undergo pre-treatment before entering the reactor. For different grades of glycerol the specific process will change, but it will generally be necessary for feeds to be purified, mixed, and heated before high purity glycerol is sent to the synthesis stage. In the GTC process, glycerol, hydrogen and methanol are mixed and heated to anywhere from 150 °C to 240 °C, at pressures between 20 and 80 atm. The preferred composition of the mixture assumes an already pure glycerol feed to be mixed, so any glycerol purchased at lower purities must be distilled to purity before entering the mixer and heater. The Lanzhou and Petroleo Brasileiro processes describe vacuum filtration and distillation of crude glycerol to remove impurities such as sodium, chloride, sulfur and phosphorous salts, fatty acids, phospholipids, glycerides, soaps and biodiesel residues. Any of these impurities can kill the catalyst used downstream. The treated glycerol purity is between 90 – 100%.
Synthesis
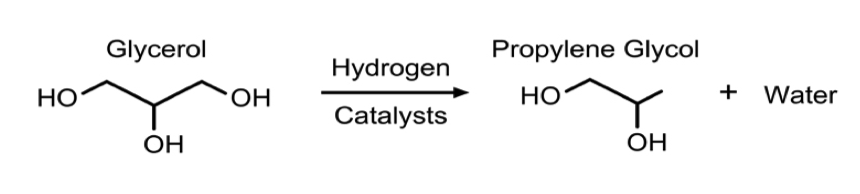
- In all process technologies considered, the basis of the synthesis is hydrogenolysis of glycerol via packed bed reactors with some form of a catalyst, usually copper based. This reaction is shown in Figure 2. In some cases, the process allows for more reactors to be used in series to achieve a higher conversion. Much of the variation in the processes being examined is based on different operating conditions and the desired purity of the product, propylene glycol.
Separation
- A series of separations is used to separate by-products from propylene glycol. Three step distillations are common; some procedures allow for additional steps, which can change the purity of the product. Common byproducts that need to be separated are methanol, acetol, water, and various other minor alcohol solutions.
Site Conditions and Capacity
In the United States, the EPA biofuel mandate for 2014 will be reduced from 18.15 billion gallons to 15-15.52 billion gallons [17], so the production of biodiesel will decrease, decreasing the supply of crude glycerol in the United States. In South America, Argentina and Brazil are the largest producers of biodiesel, with production in Brazil growing at the fastest rate. It is estimated that 25-30% of Brazilian glycerol production went to drain in 2010 and 2011, indicating a large supply of inexpensive feedstock [6]. Building a facility in Salvador da Bahia, Brazil not only enables access to this supply of inexpensive glycerol, but also provides access to a port city and thus allows export of propylene glycol to high demand markets such as China and the U.S. Additional benefits of building in Brazil include the lower corporate tax rate at 34% compared to 40% in the United States [18] and the temperate climate with an almost constant average temperature of 80 °F [19]. Dow Chemical currently operates a conventional propylene glycol facility near Salvador, indicating a potentially strong market in the area [20]. The capacity selected for this project is 10,000 ton/year. Current plants using comparable technology, such as ADM and Oleon operate at 100,000- and 200,000-tons, respectively [21]. The plant capacity is therefore relatively small, which leaves room for increased production.