Reactors
Title: Reactors
Author: Sean Cabaniss, David Park, Maxim Slivinsky and Julianne Wagoner
Steward: Fengqi You
Date Presented: February 4, 2014 /Date Revised: February 4, 2014
Introduction
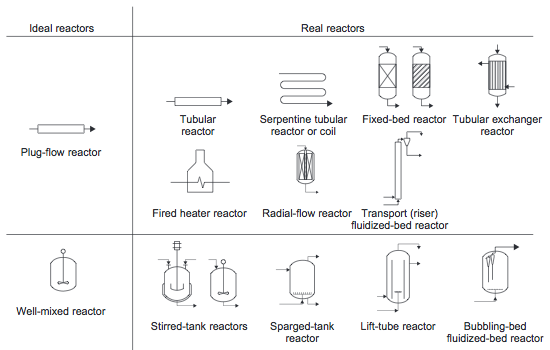
Ideal Reactors
Plug Flow Reactor (PFR)
A PFR with tubular geometry has perfect radial mixing but no axial mixing. All materials hav the same residence time, τ, and experience the same temperature and concentration profiles along the reactor. Equation for PFR is given by:
where M = molar flow rate, dV is the incremental volume, and R is the rate of reaction per unit volume.
This equation can be integrated along the length of the reactor to yield relationships between reactor resident time and concentration or conversion.
Continuously Stirred Tank Reactor (CSTR)
In a CSTR there is no spatial variation- the entire vessel contents is at the same temperature, pressure, and concentration. Therefore the fluid leaving the reactor is at the same temperature and concentration as the fluid inside the reactor.
The material balance across the CSTR is given by:
General Reactor Design
The design of the reactor should not be carried out separately from the overall process design due to the significant impact on capital and operating costs on other parts of the process[1].
Collect Required Data
Out of all process equipment, reactor design requires the most process input data: reaction enthalpies, phase-equilibrium constants, heat and mass transfer coefficients, as well as reaction rate constants. All of the aforementioned parameters can be estimated using simulation models or literature correlations except for reaction rate constant constants, which need to be determined experimentally.
Enthalpy of Reaction
The heat given out in a chemical reaction is based on the enthalpies of the component chemical reactions, which are given for standard temperature and pressure (1 atm, 25 C).
Equilibrium Constant and Gibbs Free Energy
Reaction Mechanisms, Rate Equations, and Rate Constants
Heat and Mass Transfer Properties
Select Reaction Conditions
Chemical or Biochemical Reaction
Catalyst
Temperature
Pressure
Reaction Phase
Solvent
Concentrations
Determine Materials of Construction
Determine Rate-Limiting Step and Critical Sizing Parameters
The key parameters that determine the extent of reaction must be identified by carrying out an experiment plan with a broad range of conditions. A suitable model of reaction kinetics can then be fit to the date data. Next, the designer can specify a crtical sizing parameter for the reactor. This will usually be one of the parameters given in figure 1.
In general, the rate of reaction is usually limited by the following fundamental processes. The first three have been previously discussed, but mixing will be developed in more detail.
- Intrinsic kinetics: There will usually be one slowest step that governs the overall rate.
- Mass-transfer rate: In multiphase reactions and processes that use porous heterogeneous catalysis, mass transfer can be particularly important. Often, careful experimentation will be needed to separate the effects of mass transfer and the rate of reaction to determine which is the rate-limiting step.
- Heat-transfer rate:
Preliminary Sizing, Layout, and Costing of Reactor
Estimate the Performance
Optimize the Design
Multiphase Reactors
Reactor Design for Catalytic Processes
Design of Bioreactors
Safety Considerations in Reactor Design
Capital Cost of Reactors