Reactors
Title: Reactors
Author: Sean Cabaniss, David Park, Maxim Slivinsky and Julianne Wagoner
Steward: Fengqi You
Date Presented: February 4, 2014
Introduction
Ideal Reactors
Batch Reactors
Plug Flow Reactor (PFR)
A PFR with tubular geometry has perfect radial mixing but no axial mixing. All materials hav the same residence time, τ, and experience the same temperature and concentration profiles along the reactor. Equation for PFR is given by:
where M = molar flow rate, dV is the incremental volume, and R is the rate of reaction per unit volume.
This equation can be integrated along the length of the reactor to yield relationships between reactor resident time and concentration or conversion.
Continuously Stirred Tank Reactor (CSTR)
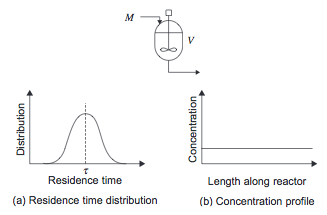
The stirred tank reactor models a large scale conventional laboratory flask and can be considered to be the basic chemical reactor. In a CSTR, shown in Figure 1, there is no spatial variation- the entire vessel contents are at the same temperature, pressure, and concentration. Therefore the fluid leaving the reactor is at the same temperature and concentration as the fluid inside the reactor.
The material balance across the CSTR is given by:
Some of the material the enters the reactor can leave immediately, while some leaves much later, so there is a broad distribution in residence time as shown in Figure 1.
Figure 1. Continuously Stirred Tank Reactor [1]
General Reactor Design
The design of the reactor should not be carried out separately from the overall process design due to the significant impact on capital and operating costs on other parts of the process[1].
Collect Required Data
Out of all process equipment, reactor design requires the most process input data: reaction enthalpies, phase-equilibrium constants, heat and mass transfer coefficients, as well as reaction rate constants. All of the aforementioned parameters can be estimated using simulation models or literature correlations except for reaction rate constant constants, which need to be determined experimentally.
Enthalpy of Reaction
The heat given out in a chemical reaction is based on the enthalpies of the component chemical reactions, which are given for standard temperature and pressure (1 atm, 25 C).
Equilibrium Constant and Gibbs Free Energy
Reaction Mechanisms, Rate Equations, and Rate Constants
Heat and Mass Transfer Properties
Select Reaction Conditions
Chemical or Biochemical Reaction
Catalyst
Temperature
Pressure
Reaction Phase
Solvent
Concentrations
Determine Materials of Construction
Determine Rate-Limiting Step and Critical Sizing Parameters
The key parameters that determine the extent of reaction must be identified by carrying out an experiment plan with a broad range of conditions. In general, the rate of reaction is usually limited by the following fundamental processes. The first three have been discussed in previous sections. Mixing will be developed in more detail in its own section.
- Intrinsic kinetics: There will usually be one slowest step that governs the overall rate.
- Mass-transfer rate: In multiphase reactions and processes that use porous heterogeneous catalysis, mass transfer can be particularly important. Often, careful experimentation will be needed to separate the effects of mass transfer and the rate of reaction to determine which is the rate-limiting step.
- Heat-transfer rate: The rate of heat addition can become the governing parameter for endothermic reactions. Heat-transfer devices such as heat exchangers or fired heaters may need to be used.
- Mixing: The time taken to mix the reagents can be the limiting step for very fast reactions.
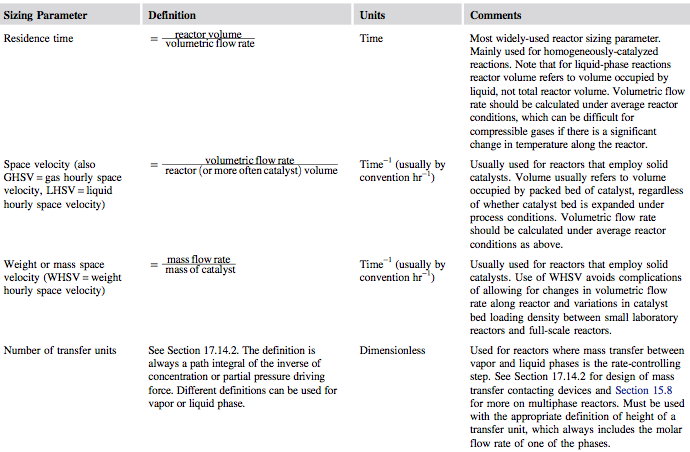
Once rate data have been collected, the designer can fit a suitable model of reaction kinetics. Next, a critical sizing parameter can be specified for the reactor. This will usually be one of the parameters given in Figure 1.
Figure 1. Reactor Sizing Parameters [1]
Preliminary Sizing, Layout, and Costing of Reactor
Estimate the Performance
Optimize the Design
Mixing in Industrial Reactors
Mixing plays an important role in many processing stages, including reactor performance. It is critical to select the appropriate method of mixing in order to ensure the process produces the desired process yields, product purity, and cost effectiveness.
Correlations such as the Reynolds number can be used to determine the extent of mixing and correlate power consumption and heat transfer to the reactor shell [2]. In some cases, simple correlations may not be adequate:
- If dead zones cannot be tolerated for reasons of product purity, safety, etc.
- If reactor internals are complex
- If reaction selectivity is very sensitive to mixing
In these cases, it is usually necessary to carry out a more sophisticated analysis of mixing:
- Use computational fluid dynamics to model the reactor
- Use physical modeling (“cold flow”) experiments
- Use tomography methods to look at performance of real reactor
Gas Mixing
Gases mix easily because of their low viscosities. The mixing given by turbulent flow in a length of pipe is usually sufficient for most purposes [1]. Orifices, vanes, and baffles can be used to increase turbulence.
Liquid Mixing
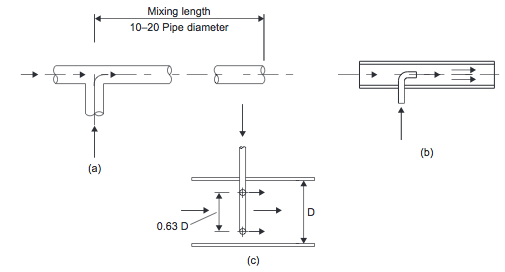
- Inline Mixing Inline mixers can be used for the continuous mixing of low-viscosity fluids. One inexpensive method involves the use of static devices that promote turbulent mixing in pipelines. Some typical designs are shown in Figures 2(a), (b), and (c).
- Figure 2. Inline mixers: (a) tee; (b) injection; (c) annular [1]
- When mixing low viscosity fluids (<50 mNs/m2) with similar densities and flow rates, a simple mixing tee, Figure 2(a), followed by a length of pipe equal to 10 to 20 pipe diameters, is suitable [1].
- When one flow is much lower than the other, an injection mixer, Figure 2(b&c), should be used. A satisfactory blend will be achieved in about 80 pipe diameters [1]. Baffles or other flow restrictions can be used to reduce the mixing length required. These mixers work by introducing one fluid into the flowing stream of the other through a concentric pipe or an annular array of jets [1].
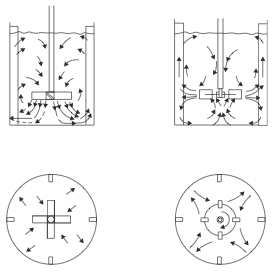
- Stirred Tanks Stirred tanks were discussed in the "Ideal Reactors" section. Mixing is conducted by an impeller mounted on a shaft driven by a motor. The reactor usually contains baffles or other internals to induce turbulence and prevent the contents from swirling and creating a vortex. Typically, baffles are 1/10 of diameter and located 1/20 of diameter from wall [2]. A typical arrangement of agitator and baffles in a stirred tank, and the flow pattern generated, is shown in Figure 3. Mixing occurs through the bulk flow of the liquid and by the motion of the turbulent eddies created by the agitator. Bulk flow is the predominant mixing mechanism required for the blending of miscible liquids and for solids suspension. Turbulent mixing is important in operations involving mass and heat transfer, which can be considered as shear-controlled processes [1].
- Figure 3. Agitator arrangements and flow patterns [1]
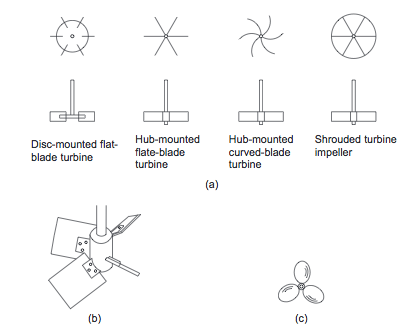
- At high Reynolds numbers (low viscosity), one of the three basic types of impeller shown in Figure 4 should be used. For processes controlled by turbulent mixing, the flat-bladed (Rushton) turbines are appropriate. For bulk mixing, the propeller and pitched-bladed turbines are appropriate [1].
- Figure 4. Basic impeller types [1]
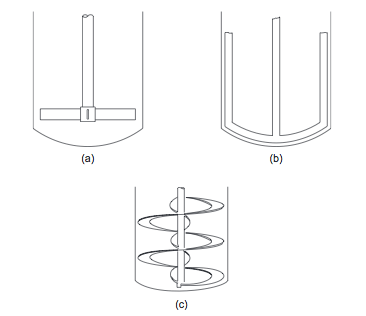
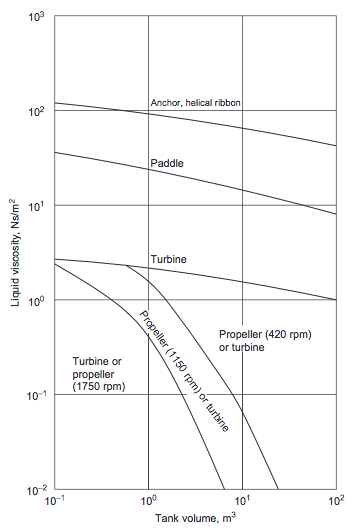
- For more viscous fluids, paddle, anchor, and helical ribbon agitators (Figures 5(a), (b), and (c)), are used [1]. The selection chart given in Figure 6 can be used to make a preliminary selection of the agitator type, based on the liquid viscosity and tank volume [1].
- Figure 5. Low-speed agitators [1]
- Figure 6. Agitator selection guide [1]
Gas-Liquid Mixing
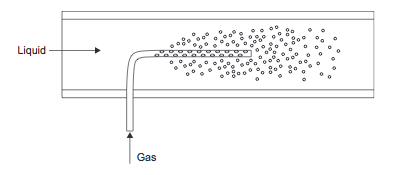
Gases can be mixed into liquids using the inline mixing or stirred tank methods discussed previously. A special type of gas injector, called a sparger (shown in Figure 7) can also be used. This is a long injection tube with multiple holes drilled in it.
Figure 7. Gas sparger [1]
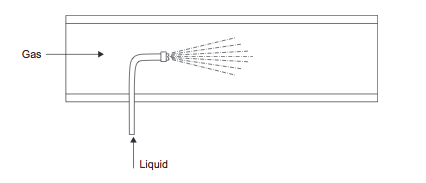
A small flow of liquid can be dispersed into a gas stream using a spray nozzle (Figure 8).
Figure 8. Liquid injection into gas [1]
Solid-Liquid Mixing
Solids are usually added to a liquid in a stirred tank at atmospheric pressure. In order to allow more accurate control of dissolved solid concentration, mixing of solids and liquids is often carried out as a batch operation [1].
Heating and Cooling of Reacting Systems
Types of Reactors
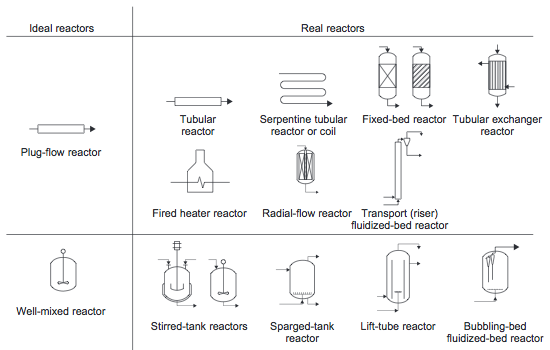
Most reactors used in industry approximate the ideal batch reactor, PFR, or CSTR. In fact, real reactors can be modeled as networks or combinations of multiple plug-flow and stirred-tank reactors [1]. Examples of real reactors that approximate the flow pattern of ideal reactors are shown in Figure 10. These reactors will be discussed in more detail in the following sections.
Figure 10. Ideal reactors and some real reactors that approximate the same flow pattern [1]
Vapor-Liquid Reactors
Vapor-liquid reactions are important in many chemical processes. For example, oxygenation and hydrogenation reactions are usually carried out with the organic component in the liquid phase [1]. If the residence time requirements are short enough, vapor-liquid contacting columns are preferred because of the high area for mass transfer. Trayed or packed columns can be used to contact vapor and liquid for reaction. Please see the separation processes section of this website for more information. The column packing may be catalytically active or could be inert packing [2]. Stirred tanks or tubular reactors are used when long residence time is needed for the liquid phase [1]. A summary of common goals for vapor-liquid reactors and the reactors used to achieve those goals is shown in Table 1.
| Goal | Types of Vapor-Liquid Reactors | Examples |
|---|---|---|
| Maintain low concentration of gas component in liquid |
|
|
| Contact gas and liquid over catalyst |
| |
| React a component out of the gas phase to high conversion | *Multi-stage V/L contactor (reactive absorption column)
|
Table 1. Summary of Vapor-Liquid Reactors
Catalytic Processes
Bioreactors
Safety Considerations in Reactor Design
Capital Cost of Reactors
Conclusions
References
- Towler, G.P. and Sinnot, R. (2012). Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. Elsevier.
- Towler, G.P. (2012). Chemical Engineering Design, PowerPoint presentation.