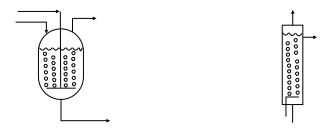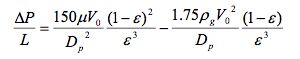Reactors
Title: Reactors
Author: Sean Cabaniss, David Park, Maxim Slivinsky and Julianne Wagoner
Steward: Fengqi You
Date Presented: February 4, 2014
Introduction
Ideal Reactors
Batch Reactors
Plug Flow Reactor (PFR)
A PFR with tubular geometry has perfect radial mixing but no axial mixing. All materials hav the same residence time, τ, and experience the same temperature and concentration profiles along the reactor. Equation for PFR is given by:
where M = molar flow rate, dV is the incremental volume, and R is the rate of reaction per unit volume.
This equation can be integrated along the length of the reactor to yield relationships between reactor resident time and concentration or conversion.
Continuously Stirred Tank Reactor (CSTR)
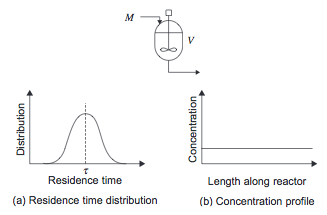
The stirred tank reactor models a large scale conventional laboratory flask and can be considered to be the basic chemical reactor. In a CSTR, shown in Figure 1, there is no spatial variation- the entire vessel contents are at the same temperature, pressure, and concentration. Therefore the fluid leaving the reactor is at the same temperature and concentration as the fluid inside the reactor.
The material balance across the CSTR is given by:
Some of the material the enters the reactor can leave immediately, while some leaves much later, so there is a broad distribution in residence time as shown in Figure 1.
Figure 1. Continuously Stirred Tank Reactor [1]
More information on stirred tanks can be found in the Mixing section.
General Reactor Design
The design of the reactor should not be carried out separately from the overall process design due to the significant impact on capital and operating costs on other parts of the process[1].
Collect Required Data
Out of all process equipment, reactor design requires the most process input data: reaction enthalpies, phase-equilibrium constants, heat and mass transfer coefficients, as well as reaction rate constants. All of the aforementioned parameters can be estimated using simulation models or literature correlations except for reaction rate constant constants, which need to be determined experimentally.
Enthalpy of Reaction
The heat given out in a chemical reaction is based on the enthalpies of the component chemical reactions, which are given for standard temperature and pressure (1 atm, 25 C).
The following equation is used to convert enthalpies from standard conditions to the process conditions:
Equilibrium Constant and Gibbs Free Energy
Reaction Mechanisms, Rate Equations, and Rate Constants
Heat and Mass Transfer Properties
Select Reaction Conditions
Chemical or Biochemical Reaction
Catalyst
Temperature
Pressure
Reaction Phase
Solvent
Concentrations
Determine Materials of Construction
Determine Rate-Limiting Step and Critical Sizing Parameters
The key parameters that determine the extent of reaction must be identified by carrying out an experiment plan with a broad range of conditions. In general, the rate of reaction is usually limited by the following fundamental processes. The first three have been discussed in previous sections. Mixing will be developed in more detail in its own section.
- Intrinsic kinetics: There will usually be one slowest step that governs the overall rate.
- Mass-transfer rate: In multiphase reactions and processes that use porous heterogeneous catalysis, mass transfer can be particularly important. Often, careful experimentation will be needed to separate the effects of mass transfer and the rate of reaction to determine which is the rate-limiting step.
- Heat-transfer rate: The rate of heat addition can become the governing parameter for endothermic reactions. Heat-transfer devices such as heat exchangers or fired heaters may need to be used.
- Mixing: The time taken to mix the reagents can be the limiting step for very fast reactions.
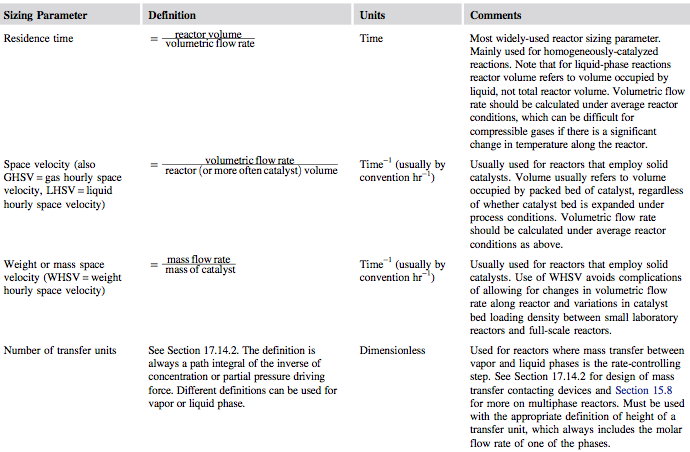
Once rate data have been collected, the designer can fit a suitable model of reaction kinetics. Next, a critical sizing parameter can be specified for the reactor. This will usually be one of the parameters given in Figure 1.
Figure 1. Reactor Sizing Parameters [1]
Preliminary Sizing, Layout, and Costing of Reactor
Estimate the Performance
Optimize the Design
Mixing in Industrial Reactors
Mixing plays an important role in many processing stages, including reactor performance. It is critical to select the appropriate method of mixing in order to ensure the process produces the desired process yields, product purity, and cost effectiveness.
Correlations such as the Reynolds number can be used to determine the extent of mixing and correlate power consumption and heat transfer to the reactor shell [2]. In some cases, simple correlations may not be adequate:
- If dead zones cannot be tolerated for reasons of product purity, safety, etc.
- If reactor internals are complex
- If reaction selectivity is very sensitive to mixing
In these cases, it is usually necessary to carry out a more sophisticated analysis of mixing:
- Use computational fluid dynamics to model the reactor
- Use physical modeling (“cold flow”) experiments
- Use tomography methods to look at performance of real reactor
Gas Mixing
Gases mix easily because of their low viscosities. The mixing given by turbulent flow in a length of pipe is usually sufficient for most purposes [1]. Orifices, vanes, and baffles can be used to increase turbulence.
Liquid Mixing
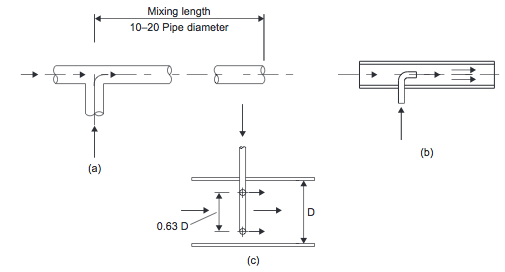
- Inline Mixing Inline mixers can be used for the continuous mixing of low-viscosity fluids. One inexpensive method involves the use of static devices that promote turbulent mixing in pipelines. Some typical designs are shown in Figures 2(a), (b), and (c).
- Figure 2. Inline mixers: (a) tee; (b) injection; (c) annular [1]
- When mixing low viscosity fluids (<50 mNs/m2) with similar densities and flow rates, a simple mixing tee, Figure 2(a), followed by a length of pipe equal to 10 to 20 pipe diameters, is suitable [1].
- When one flow is much lower than the other, an injection mixer, Figure 2(b&c), should be used. A satisfactory blend will be achieved in about 80 pipe diameters [1]. Baffles or other flow restrictions can be used to reduce the mixing length required. These mixers work by introducing one fluid into the flowing stream of the other through a concentric pipe or an annular array of jets [1].
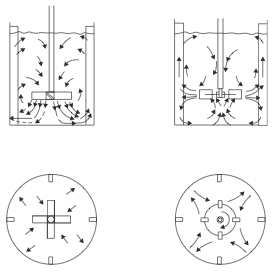
- Stirred Tanks Stirred tanks were discussed in the Ideal Reactors section. Mixing is conducted by an impeller mounted on a shaft driven by a motor. The reactor usually contains baffles or other internals to induce turbulence and prevent the contents from swirling and creating a vortex. Typically, baffles are 1/10 of diameter and located 1/20 of diameter from wall [2]. A typical arrangement of agitator and baffles in a stirred tank, and the flow pattern generated, is shown in Figure 3. Mixing occurs through the bulk flow of the liquid and by the motion of the turbulent eddies created by the agitator. Bulk flow is the predominant mixing mechanism required for the blending of miscible liquids and for solids suspension. Turbulent mixing is important in operations involving mass and heat transfer, which can be considered as shear-controlled processes [1].
- Figure 3. Agitator arrangements and flow patterns [1]
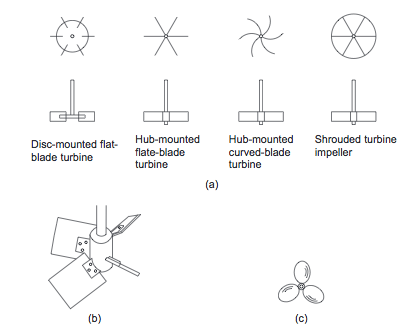
- At high Reynolds numbers (low viscosity), one of the three basic types of impeller shown in Figure 4 should be used. For processes controlled by turbulent mixing, the flat-bladed (Rushton) turbines are appropriate. For bulk mixing, the propeller and pitched-bladed turbines are appropriate [1].
- Figure 4. Basic impeller types [1]
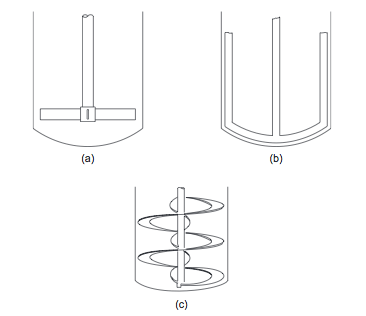
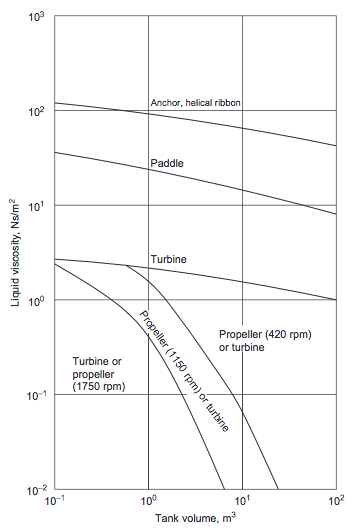
- For more viscous fluids, paddle, anchor, and helical ribbon agitators (Figures 5(a), (b), and (c)), are used [1]. The selection chart given in Figure 6 can be used to make a preliminary selection of the agitator type, based on the liquid viscosity and tank volume [1].
- Figure 5. Low-speed agitators [1]
- Figure 6. Agitator selection guide [1]
Gas-Liquid Mixing
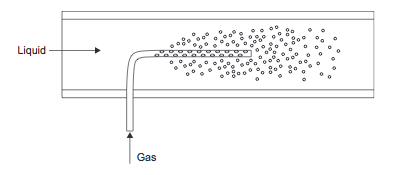
Gases can be mixed into liquids using the inline mixing or stirred tank methods discussed previously. A special type of gas injector, called a sparger (shown in Figure 7) can also be used. This is a long injection tube with multiple holes drilled in it.
Figure 7. Gas sparger [1]
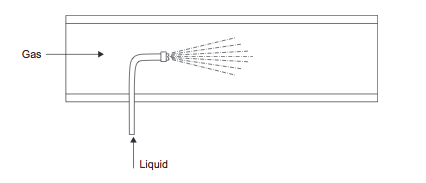
A small flow of liquid can be dispersed into a gas stream using a spray nozzle (Figure 8).
Figure 8. Liquid injection into gas [1]
Solid-Liquid Mixing
Solids are usually added to a liquid in a stirred tank at atmospheric pressure. In order to allow more accurate control of dissolved solid concentration, mixing of solids and liquids is often carried out as a batch operation [1].
Heating and Cooling of Reacting Systems
Types of Reactors
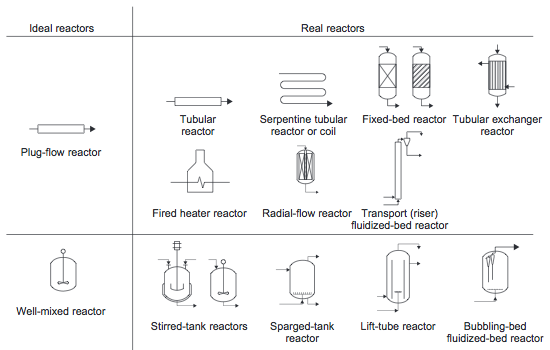
Most reactors used in industry approximate the ideal batch reactor, PFR, or CSTR. In fact, real reactors can be modeled as networks or combinations of multiple plug-flow and stirred-tank reactors [1]. Examples of real reactors that approximate the flow pattern of ideal reactors are shown in Figure 10. These reactors will be discussed in more detail in the following sections.
Figure 10. Ideal reactors and some real reactors that approximate the same flow pattern [1]
Vapor-Liquid Reactors
Vapor-liquid reactions are important in many chemical processes. For example, oxygenation and hydrogenation reactions are usually carried out with the organic component in the liquid phase [1]. A summary of common goals for vapor-liquid reactors and the reactors used to achieve those goals is shown in Table 1.
| Goal | Types of Vapor-Liquid Reactors | Examples |
|---|---|---|
| Maintain low concentration of gas component in liquid |
|
|
| Contact gas and liquid over catalyst |
|
|
| React a component out of the gas phase to high conversion |
|
|
Table 1. Summary of Vapor-Liquid Reactors [2]
If the residence time requirements are short enough, vapor-liquid contacting columns are preferred because of the high area for mass transfer. Trayed or packed columns can be used to contact vapor and liquid for reaction. The column packing may be catalytically active or could be inert packing [2]. Please see the separation processes section of this website for more information on the types of processes used for the third goal listed.
Stirred tanks or tubular reactors are used when long residence time is needed for the liquid phase [1]. These types of reactors and more will be discussed in the catalytic processes section of this page.
The reactors listed under the first goal in the table are unique to vapor-liquid processes. The basic concept of a sparger was discussed in the mixing section. Sparged reactors are shown in Figure 11.
Figure 11. Sparged stirred tank and tubular reactors [2]
The gas is bubbled up through the liquid in a sparged reactor. For smaller bubbles, a porous pipe diffuser can be used instead [2]. The designer must allow some disengaging space at the top of the reactor, or entrainment will be excessive. If the gas flow rate is large then the gas flow can be used as the primary means of agitation. Perry's Handbook suggests the following air rates (ft3/ft2min) for agitating an open tank full of water at 1 atm:
| Degree of agitation | Liquid depth 9 ft | Liquid depth 3 ft |
|---|---|---|
| Moderate | 0.65 | 1.3 |
| Complete | 1.3 | 2.6 |
| Violent | 3.1 | 6.2 |
Table 2. Summary of suggested flow rates for gas flow as agitation [2]
Catalytic Processes
A catalyst increases the rate of a chemical reaction without itself becoming permanently changed by the reaction. Catalysts allow reactions to be run in smaller reactors and operated at lower temperatures and improve selectivity. Therefore, catalysts will almost always lead to a more economically attractive process than a noncatalytic route. [1] Catalysts are normally selected based on performance rather than price since increases catalysts selectivity will almost always quickly pay back any price premium expected by the manufacturer. It is important to test the catalysts under conditions that are representative of process conditions [1].
Catalyst activity often deteriorates over time [2]. Common causes of deactivation include:
- Poisoning by components in feed (e.g. base destroys acid catalyst)
- Blockage of pores or active sites by byproducts such as coke
- Thermal or hydrothermal modification of catalyst structure
Slow activity loss can be compensated by:
- Putting in more catalyst (lower space velocity)
- Slowly raising reactor temperature
Rapid activity loss may require moving the catalyst to a continuous regeneration zone [2].
Catalytic reactions can be either homogenous (catalyst is in the same phase as the reagents) or heterogeneous (catalyst is not in the same phase as the reagents).
Homogeneous Catalysis
- Homogeneous catalysis can be conducted in the basic batch reactors, PFRs, or CSTRs that have already been discussed. However, when the catalyst is in the same phase as the reagent, recovering this catalyst after the reaction can be difficult and expensive, particularly if the catalyst is sensitive to high temperatures [2]. Providing adequate interfacial area is also a challenge of homogeneous catalysis. A reaction often only occurs at the interface or in the boundary layer between the catalyst and the reagents. Increased mixing can increase the rate and selectivity of the reaction, but this can require detailed and expensive mixing equipment [2]. For these reasons, reactions requiring homogenous catalysts are not usually used unless an easy separation can be found to recover the catalyst.
Heterogeneous Catalysis
- Catalyst recovery in processes involving heterogeneous catalysis is much easier. However, the rate of reaction is limited by the available inter-phase surface area and the mass transfer of reagents and products to and from the interface [2]. Therefore, reactors for these processes are design to reduce these limitations.
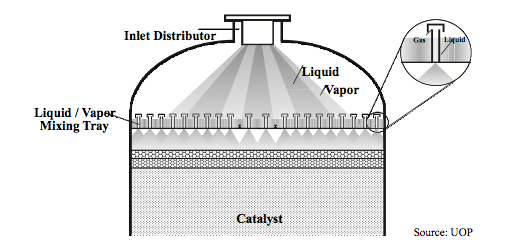
- Fixed Bed Reactors
- In a fixed-bed reactor, the reagent flows over a stationary bed of packed catalyst [1]. This is the most common type of reactor used for heterogeneous catalysis as long as the catalyst does not require continuous regeneration and the reaction mixture does not require high agitation [2]. The amount of catalyst necessary can be found using the following equations:
- The ratio of the bed height (L) to the diameter (D) determines the distribution of reagents and the pressure drop across the bed. An increased L/D ratio creates a more even distribution and less change of localized deactivation or "hot spots." However, increasing the L/D ratio increases the pressure drop, requiring higher compression and pumping costs [2]. The Ergun equation can be used to calculate the pressure drop in packed beds.
- Where V is the superficial velocity (volume flowrate divided by cross-sectional area), μ is the viscosity, Dp is the particle diameter and ε is the porosity of the packed bed [2]. Given these trade-offs, it may make sense to split the catalyst over several beds [2].
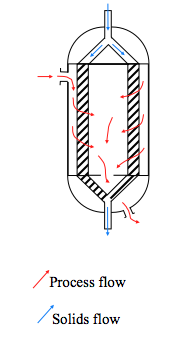
- Radial Flow Reactors
- When there is very little pressure drop available, the L/D ratio must be much less that one [2]. A common solution to this is to use a radial flow reactor with the catalyst contained in an annulus between vertical perforated or slotted screens. The fluid flows radially through the bed and the direction of flow can be either inwards or outwards [1]. An example of a radial flow reactor is shown in Figure 12.
- Figure 12. Radial flow reactor [2]
- Moving Bed Reactors
- A moving bed reactor is similar to a radial flow reactor, but the catalyst is moved through the annular space [2].
- Fluidized Bed Reactors
- If the fluid flow is up through the catalyst bed then the bed can become fluidized if the pressure drop is high enough to support the weight of the catalyst. Fluidized beds usually have a lower pressure drop than down flow at high flow rates [2]. In addition, fluidizing the catalyst eases the transition from one reaction zone to another.
- The catalyst bed is fluidized using a distributor to inject fluidization fluid, which is not necessarily the feed. Fluidization occurs when the bed pressure drop balances the weight of the particles, or
- Where ∆P is the pressure drop, ρp and ρg are the densities of the particle and gas respectively, εm is the porosity at minimum fluidization, and L is the height of the bed [2]. Fluidization can only be used with relatively small sized particles (<300 micrometers with gases). The solid material must be strong enough to withstand attrition in the fluidized bed and cheap enough to allow for make-up to replace attrition losses [1]. A fluidized-bed reactors must also make allowance for separating the fluid-phase product from entrained solids so that solids are not carried out of the reactor [1].
- Trickle Bed Reactors
- Trickle bed reactors are used when all three phases are involved in the reaction. They must ensure good distribution of both the vapor and the liquid, without channeling of either phase [2]. In a trickle bed reactor, the liquid flows down over the surface of a stationary bed of solids. The gas phase usually also flows downwards with the liquid, but countercurrent flow is feasible as long as flooding conditions are avoided [1]. This requires a more sophisticated distributor like those used for packed distillation columns [2]. An example of a trickle bed reactor is shown in Figure 13.
- Figure 13. Example of trickle bed reactor [2]
- Slurry Reactors
- Liquid is mixed up in the liquid in slurry phase reactions. Slurry reactors are prone to attrition of the solids, caused by pumping or agitation of the liquid [1]. Slurry-phase operation is usually not preferred for processes that use heterogeneous catalysts because the catalyst tends to become eroded and can be difficult to recover from the liquid [1].
Bioreactors
Enzyme Catalysis
Cell Growth
Types of Bioreactors
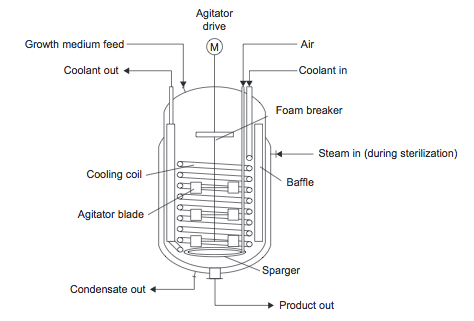
- Stirred Tank Fermenter
- Figure 14. Fermentation reactor [1]
- Shaftless Bioreactors
Safety Considerations in Reactor Design
Capital Cost of Reactors
Conclusions
References
- Towler, G.P. and Sinnot, R. (2012). Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. Elsevier.
- Towler, G.P. (2012). Chemical Engineering Design, PowerPoint presentation.