Utility systems
Authors: David Chen, [2014] Joshua Lee, [2015] Brett Sleyster, [2016] and Tom Aunins [2016]
Stewards: David Chen, Jian Gong, and Fengqi You
Date Presented: January 13, 2014 /Date Revised: January 14, 2014
Introduction
Many chemical processes do not take place at ambient temperature or pressures. In order to reach these non-ambient conditions, utilities will have to be used to raise or lower temperatures and compress gases. (Towler, Towler/UOP) Utilities often contribute 5 to 10% of the price of a product, and may come from public or private utility companies or on-site plants. For purchased utilities, costs depend on consumption, while for company-owned utilities, there will be both capital and operating costs. They include things such as steam for heating, electricity, cooling water, refrigeration, fuels such as natural gas, wastewater treatment, waste disposal, and landfill. Steam is often the largest utility cost. Cogeneration unit can supply electricity accompanied with different steam pressures. (Seider 2009)
Electricity
Electricity is used to power many different kinds of equipment. It has many advantages: it is efficient (> 90%), reliable, available in a wide range of power, shaft speeds, designs, lifetimes, convenience, costs, and maintenance. It is generally used up to 200 hp, and sometimes over 10,000 Hp. In chemical process plants, the electricity demand is generally determined by the work or energy required for compression, pumping, air cooling, lights, and many other items. This electricity often times is purchased from local electricity providers, but many plants generate their own electricity via sophisticated processes.
Electricity is rarely used as a primary heat utility in large-scale chemical plants for a variety of reasons. The main disadvantages of using electricity as a heat utility are as follows (Towler 2012):
- Heat from electricity is two to three times more expensive than heat from fuels. This is attributed to the lack of efficiency when creating heat from electricity.
- Electrical heating units are expensive, require high maintenance, and must comply with strict safety regulations.
- Electrical heating units are unsafe compared to steam heating units. In steam systems, the physically steam controls the temperature, whereas in electrical heating units temperature is controlled by temperature controllers, which can fail or burn out.
The use of electricity carries with it some hazards depending on the environment. Extra care must be taken when using electrically-powered equipment in areas which may have combustible fluids, vapors, or dust, and where liquids may be present. (Seider 2009 pg 606)
Conventional Power Station
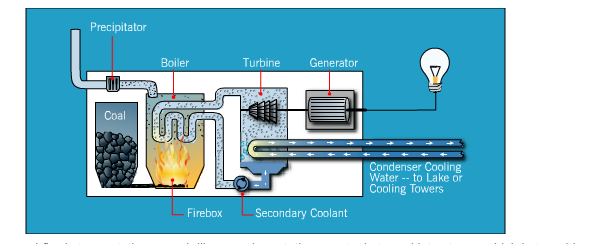
In general, most electricity is generated from a conventional coal-fired process, whether it be on-site or purchased from a provider. Coal-fired processes have been used to create electricity throughout history, and technological advances have increased its efficiency and use worldwide. In a coal-fired steam station—much like a nuclear station—water is turned into steam, which in turn drives turbine generators to produce electricity. There are several variations on how to create energy from coal, but here are the basics of how a coal-fired process works:
- Heat is created:
- Before the coal is burned, it is pulverized to the fineness of talcum powder. It is then mixed with hot air and blown into the firebox of the boiler. Burning in suspension, the coal/air mixture provides the most complete combustion and maximum heat possible.
- Water turns to steam:
- Highly purified water, pumped through pipes inside the boiler, is turned into steam by the heat. The steam reaches temperatures of up to 1,000 degrees Fahrenheit and pressures up to 3,500 pounds per square inch, and is piped to the turbine.
- Steam turns the turbine:
- The enormous pressure of the steam pushing against a series of giant turbine blades turns the turbine shaft. The turbine shaft is connected to the shaft of the generator, where magnets spin within wire coils to produce electricity.
- Steam is converted back to water:
- After doing its work in the turbine, the steam is drawn into a condenser, a large chamber in the basement of the power plant. In this important step, millions of gallons of cool water from a nearby source (such as a river or lake) are pumped through a network of tubes running through the condenser. The cool water in the tubes converts the steam back into water that can be used over and over again in the plant.
- Repeat:
- The cooling water is returned to its source without any contamination, and the steam water is returned to the boiler to repeat the cycle.
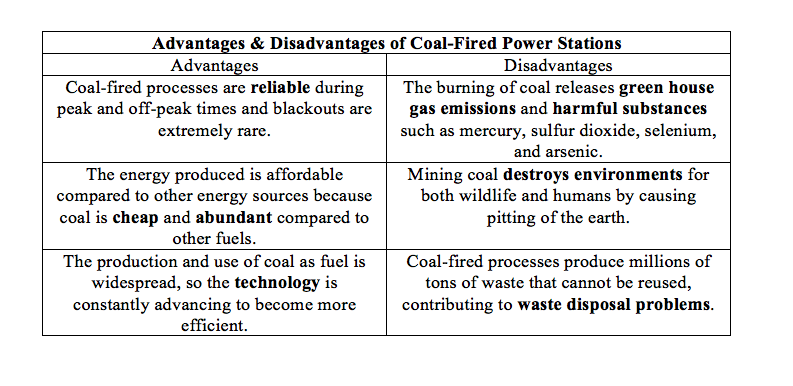
Advantages & Disadvantages of Coal-Fired Energy Production
Gas-Turbine Cogeneration Process
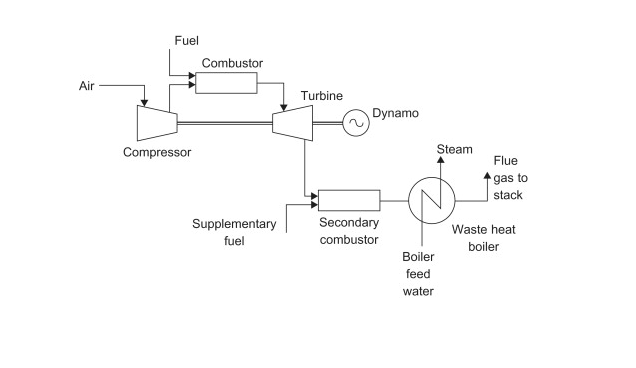
When generating energy on-site, many plants use a gas-turbine cogeneration process. The thermal efficiency of a gas-turbine process is in the range of 70-80% while conventional power stations, such as coal-fired processes, have a 30-40% efficiency. The lower efficiency in more conventional power stations is attributed to wasted heat in the exhaust steam in the condenser. One example of a gas-turbine process is outlined in the following figure. Figure 3.1 is a gas-turbine cogeneration process with a heat recovery steam generator (waste-heat) boiler.
Overall, the process illustrated is not much different from a coal-fired process. The main differences are that the cogeneration process creates both electricity and a heat utility, and cogeneration processes use natural gas instead of coal. Many of the advantages and disadvantages are similar to those of the coal-fired process, but the cogeneration has a much higher efficiency, creates heat to be used in another process, and uses a more volatile and expensive fuel. The main advantage of cogeneration over coal-fired energy production is that heat is not wasted. In coal-fired processes, heat is released and wasted during electricity generation. Cogeneration captures some, if not all of the byproduct for heat, which is an extremely useful utility that will be discussed in subsequent sections. In summary, the cogeneration plant is superior to the coal-fired process because of its higher efficiency and ability to create a useful heat utility.
Obviously any engineer would design the cogeneration plant to meet at least the energy requirement necessary for plant operation, but cogeneration plants often times are designed to exceed the plant electricity requirement to drive another source of capital. Many describe this scenario as a "make or buy" scenario (Towler 2012). This scenario provides chemical producers leverage when negotiating contracts with outsourced electricity providers and this allows plants to purchase electricity at a wholesale price. This is a huge advantage of considering on-site electricity production because electricity is needed in relatively high quantities for all chemical plants. Being able to minimize electricity costs, or even profit off of electricity production is a huge economical consideration that all plants employ.
Process Heating
The key objective of this section is to discuss how processes are heated. Heating utilities are necessary for proper usage of distillers, reactors, condensers, and several other integral types of equipment. More specifically, steam, fired heat, and hot oil/specialized heat transfer fluids will be discussed in the following subsections.
Steam
Steam is the most commonly used heat utility used in chemical plants, and as a result understanding how it is used is essential in the study of Utility systems. Steam is used both as a process fluid (feedstock, diluent to absorb heat of reaction, heating agent, and stripping agent in absorbers and adsorbers ) and utility. It can be used to drive pumps and compressors, ejectors (for producing a vacuum), and heat exchangers. As one can clearly see, steam is a versatile, and useful utility.
Here are a few advantages of using steam as opposed to other methods of process heating (Towler 2012):
- By controlling the pressure of the steam, one can control the temperature at which the heat is released. Having a strong control over the temperature is essential in several processes. =
- Steam is an efficient heat source because the heat of condensation of steam is very high. Meaning that there is is high output per mass of utility at a constant temperature.
- Heat exchangers that use steam are relatively cheap because condensing steam has a high heat transfer coefficient.
- Steam is nonflammable, nontoxic, and inert to several process fluids.
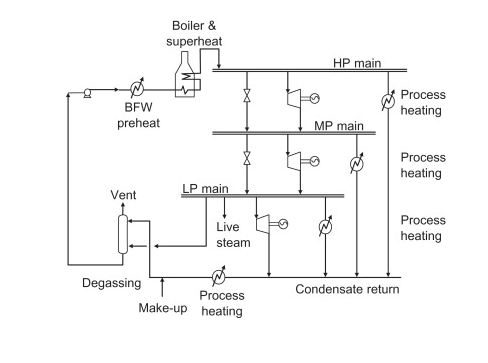
Chemical plants generally have a network of pipelines exclusively for providing steam. These networks generally have steam at a low pressure, a medium pressure, and a high pressure. The image below illustrates a basic steam system.
In the diagram above, boiler feed water at a high pressure is preheated and fed to other boilers. These other boilers superheat the steam to create a high pressure and high temperature steam stream. The steam is superheated past the dew point to account for heat loss in the pipelines. A portion of the high pressure steam is used for process heating in areas of the plant that require high temperatures. The rest of the high pressure steam is turned into medium pressure steam by valves and steam turbines. The medium pressure steam is then used to heat medium temperature processes and to form low pressure steam. The low pressure steam can be used to heat low pressure processes and it can be expanded in condensing turbines to create shaft work and energy. In summary, steam can be used for an innumerable amount of action items in a plant. High pressure, medium pressure, and low pressure steam can all be used as a heat source. Low pressure steam has utilities in creating electricity and it also has several other uses.
Fired Heat
In many cases, processes in a plant require a heat source stronger than high pressure and temperature steam. That is when fired heat is used, which is generally at temperatures above 523K. Streams can be heated directly in the furnace tubes or via a hot oil circuit or heat transfer fluid, which will be discussed in detail in the next section. Most fired heaters use natural gas as fuel because it burns cleaner than fuel oil. A cleaner burning fuel is always advantageous due to environmental and safety concerns. Furthermore, natural gases usually result in less wear and tear in burners and fuel lines.
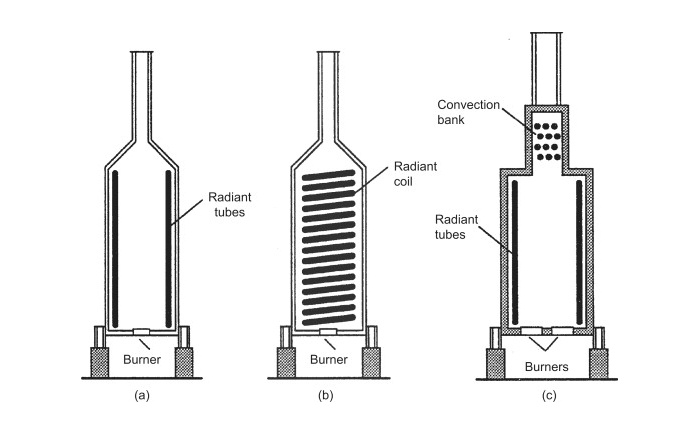
Depending on the application of the fired-heater, different design specifications can be implemented to make the fired-heater as efficient as possible. The basic construction of a fired heater starts with a cylindrical chamber that is lined with with refractory bricks. The stream that is to be heated flows through tubes inside of the furnace. These tubes can be arranged in several different arrangements such as, around the walls of the furnace, or in horizontal or vertical banks (Towler 2012). The figure below illustrates the basic construction of the fired-heater and varying tube arrangements.
Fuel is burned to heat the entire furnace, and the heat transfer occurs from the combustion gases inside of the furnace across the tubes that are filled with our desired stream. The heat transfer between the tubes and the furnace is accomplished primarily via radiation. Modern designs take advantage of convective heat transfer by adding a smaller section on top of the fired-heater where the combustion gases flow over banks of tubes as seen in (c) in the figure above. Heat transfer can be further improved via convection by adding fins or pins in the combustion section.
The cost of fired heat can be calculated by the cost of fuel fired. Natural gas and heating oil are traded as commodities and prices can be found at many online trading sites or business news sites (i.e., www.cnn.money.com). Past historic prices for forecasting can be found in the Oil and Gas Journal or from the U.S. Energy Information Adminstration (www.eia.gov).
Fuels
Fuel is burned in utility facilities such as boilers, electricity generation, and cogeneration, and can be in solid, liquid, or gas form. It can also be burned to provide heating for a process or stream or to drive pumps and compressors. The fuel is usually burned with excess air to ensure complete combustion.
A way of quantifying the amount of heat generated is by using the heating values. Higher heating value (HHV) and the lower heating value (LHV) are used. The heating is the total heat evolved by complete combustion of a fuel with dry air with both at 60 ⁰F and the flue gas after combustion brought back down to ⁰F. If the water vapor in the flue gas is not condensed, we obtain the LHV. If the water vapor is condensed, the value of heat evolved is a bit higher, and this is the HHV. Heating values for solids and liquids are usually on a per-mass basis, and gases on a per-volume basis. A specified amount of heating cannot be met with the amount of fuel calculated using only the HHV. There will be heat losses, the flue gas temperature will be greater than 60 ⁰F, and water in the flue gas will typically be vapor. (Seider 608)
Hot Oil/Specialized Heat Transfer Fluids
Specialized heat transfer fluids and hot oil circuits are used as heat sources when steam and fired heat is not appropriate. Specialized heat transfer fluids and hot oil circuits are extremely versatile in that they can be used in the temperature range of 323K to 673K. This range however is quite variable. For hot oils, the upper temperature limit is gauged based off of the thermal decomposition of the oil and coking/fouling of heat exchanger tubes.
Hot oil circuit systems are most commonly used when the plant has several small temperature heating requirements because it is more economically sound. Rather than having several fired heaters heat each small temperature requirement, it is much more economical to have one fired heater heat the hot oil and circulate that oil through each of the process to meet all of the heat needs. Hot oil systems are also generally favored over high pressure steam in processes that involve high pressure differentials between the process stream and high pressure steam. Hot oil systems are favored in this scenario because of safety concerns. If the steam were to leak, the pressure drop could cause serious safety issues.
Mineral oils are the most commonly used heat transfer fluids, and one prominent example is Dowtherm A. Dowtherm A is a combination of 26.5 wt% diphenyl in diphenyl oxide (Towler 2012) and is extremely thermally stable. These mineral oil systems generally require high flow rates.
Process Cooling
Cooling Water
Cooling water is used to cool and/or condense streams. Cooling water is usually circulated between process heat exchangers and a cooling tower. Water is cooled during downward motion by contact with air blown upwards, which can bring the water temperature to come within ~ 5 ⁰F of air’s wet-bulb temperature.Approximately 80% of the temperature reduction is due to evaporation of the cooling water and heat transfer to the surrounding air. Water can also be cooled in spray ponds and cooling ponds. Both work by providing high area for water to exchange heat with air. Water in cooling towers is lost through drift and blowdown, and makeup is usually 1.5 to 3% of the circulating rate. If a large natural body of water is nearby, it can be used as a source of cooling water and discharged downstream. This water is usually filtered to remove salts and impurities that may lead to fouling, but it is not treated.
Refrigeration
Cooling water can usually be used to cool a stream to ~ 100 ⁰F. Air can only cool to ~ 120 ⁰F. Air may be used in places where water is scarce or more costly to transport. To cool or condense streams below 100 ⁰F, a refrigerant is typically used. Chilled brine can also be used, but is less common.
Until 1995, CFC Freon R-12 (dichlorodifuloromethane) and HCFC Freon R-22 (chlorodifuloromethane) were commonly used refrigerants. However, the chlorine atom in the molecules caused the depletion of the ozone layer. The U.S. Clean Air Act Amendments of 1990 went into effect in 1995, and the production of these refrigerants has since stopped or been greatly reduced.
Cost estimates are based on ton-day of refrigeration, where a ton is the heat that needs to be removed to freeze 1 ton per day of water at 32 ⁰F. Substitutes have since been developed. R-134a is often used in place of R-12. According to the EPA, R-134a is not combustible at ambient conditions, and is essentially non-toxic under 400 ppm, and is not ozone-depleting. (Seider pg 607)
Energy Efficiency
One of the chief concerns in selecting and designing process utility systems for heating and cooling is how to achieve the most energy efficient design. There are countless means by which plants lose energy, two of the foremost being through the mixing of different temperature or pressure streams and through the disposal of warmed cooling water. (Seider, 2009) Proper utilities design can help mitigate each of these losses as well as many others. The energy efficiency of a plant will depend primarily on the heating and cooling methods that are being used and the overall system design itself. These two parameters are important in determining how well energy is transferred to the desired media as well as how well that energy is recovered and recycled.
Utility Efficiency
As mentioned above, the most commonly used utilities for process heating in large scale processes are steam, fired heat, and hot oil heaters. Of these, steam is the most commonly used. Electricity, while efficient at creating power, is not a viable source of heat in large industrial processes. Common ranges of heating efficiency for these three methods are displayed in Table 1. (Towler and Sinnott, 2012)
| Process Heating Method | Typical Efficiency |
|---|---|
| Steam (via package boiler) | 80-90% |
| Fired Heat w/ Convective Section | 85% |
| Fired Heat w/o Convective Section | 60% |
| Hot Oil Heaters/Vaporizers | 80-85% |
Efficiency in cooling processes depends more on the method used, and by extension the amount of coolant needed. Water and air utility efficiencies depend primarily on the fluid flow required to maintain the system at a desired temperature, while powered refrigeration utilities (for colder processes) have efficiencies at approximately 60%--but ranging up to 90%--of Carnot cycle efficiency, a metric of ideal refrigeration efficiency. (Towler and Sinnott, 2012) Cooling systems represent by definition a loss of energy from the main process to the utility stream, and as such it is often useful to find other uses for the heated media before discharge.
Energy Recovery
Recovery and recycle of energy is perhaps the most important aspect of creating an energy efficient plant design, and it is important for process engineers to fully consider possibilities for heat recovery in order to aid in economic viability.
Process Heat Exchange
Heat exchanger networks are a very common energy recovery method in industrial processes. These networks most frequently allow energy from heated product streams to be transferred to feed streams that must be brought up to process temperature. (Biegler, 1997) More information on the function and design of heat exchanger networks can be found on the heat exchanger wiki page. The following are several examples of energy recovery via heat exchange that are used in industrial processes.
In distillation columns the bottoms and distillate effluents have the potential for energy exchange. Though the condenser at the top of the column cannot supply its waste heat to the reboiler due to their respective temperatures, the effluent streams can supply heat to the feed via a feed-effluent exchanger. This reduces the utility requirements to raise the feed to column temperature. (Biegler, 1997)
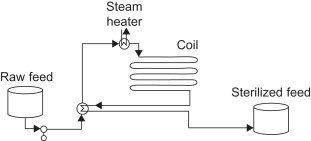
Feed sterilization, commonly used in the food industry, is a common application for heat recovery through process stream heat exchange. In this application, the feed must be heated for a certain amount of time to kill any biological contaminants, after which it can be used to heat the new raw feed for sterilization. This reduces energy demands on the steam heater and thus reduces cost. (Towler and Sinnott, 2012)
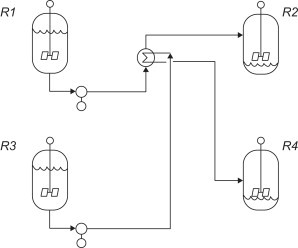
In multi-vessel batch processes it can be advantageous to exchange heat as the process fluid is being transferred between vessels. Like the previous examples, this reduces the utility needed to bring the colder feed up to process temperature, thus reducing costs. (Towler and Sinnott, 2012)
Utility Regeneration
When recovery of waste heat via transfer to other process streams is inconvenient or impossible, energy efficiency can still be improved through the regeneration of utilities. This is commonly done through the regeneration of steam by either removing heat from exiting streams or from highly exothermic reactions. Waste heat in exiting streams is removed via heat recovery steam generators (HRSGs), and is most often used on exiting flue gas and exiting process gas streams. Heat recovery from reactions is a viable option when the reactor temperature will be at 150 C or above, as this will create steam at high enough pressure to be used in other processes. (Towler and Sinnott, 2012)
Furthermore, there is opportunity for energy recovery in the expansion of compressed gas through a turbine to create electricity, a process that can be economically viable given sufficiently high flows or pressure. Such technology has been used in processes to synthesize ammonia, perform air separations, and synthesize nitric acid. (Towler and Sinnott, 2012) Recently, however, there has been a particularly strong interest for energy recovery in the natural gas industry, when gas is decompressed from major pipelines to residential low-pressure piplines. A 2001 study estimated that there is the potential to recover 21 TWh, representing 11% of natural gas transport energy, via gas expansion. (Lehman)
Process water and boiler-feed water
Process water is water that will be directly used in the process. Boiler-feed water (BFW) is used to produce steam. Both may need to be purified to prevent impurities from contaminating a process or from foul equipment. It can be used as a cooling stream when the temperature of the stream to be cooled is greater than ~300 ⁰F. Cost of BFW can be partially offset by the steam credit.
Process water that undergoes moderate pretreatment can cost ~ $0.75/1,000 gal.
Extensive treatment ~ $6.00/1,000 gal.
Sterilized for pharmaceutical processes ~ $550/1,000 gal. (Seider pg 608)
Demineralized Water
In demineralized water, minerals have been removed by ion exchange. In boiler feed water, this is to prevent salt deposition, corrosion, formation of foam, and sluicing. In process water, the ions may contaminate the process.
Waste Treatment
Most chemical processes will produce some sort of waste. Disposal occurs to the atmosphere (in the case of some gases), sewers, body of water, or a landfill. Waste may require some treatment before disposal to meet regulations. Depending on process economics, byproducts may be recovered and processed. (Seider 2009 pg 609)
Wastewater Treatment
The United States EPA regulates industrial wastewater disposal through the Clean Water Act, introduced in 1948 as the Federal Water Pollution Control Act and amended to its current form in 1972. The sweeping 1972 amendments allowed the EPA to prevent industries and persons from discharging contaminated water into fresh water sources and set water quality standards. (Summary of the Clean Water Act) In accordance with this law, process plants in the United States treat wastewater at on-site or near-site treatment centers before releasing it into the surrounding environment.
Wastewater effluent streams, along with water runoff from around the plant, are treated to control for pH, toxicity, suspended solids, and biological oxygen demand (for aquatic life protection) prior to discharge. Each of these controls is typically addressed with a separate method. Acidity and basicity is balanced through the addition of an acid or alkaline solution. Toxic wastewater may be treated with chemical processes or simply diluted to safe concentrations. Suspended solids can be removed via filtration and/or with clarifiers. Oxygen demand of wastewater can be mitigated using activated sludge treatment processes. Once the water quality complies with the EPA, and state-mandated, regulations, it can be safely released. More information on the large number of industry-specific guidelines for waste effluent can be found on the EPA website (http://www.epa.gov/eg/industrial-effluent-guidelines).
Air-Pollution Management
Introduction
In the United States air pollution is regulated in the Clean Air Act, and almost all pollutant emitting plants are regulated under this law. The types of plants that can release significant emissions include petroleum refineries, sulfur recovery plants, carbon-black plants, fuel conversion plants, chemical process plants, fossil fuel plants, and petroleum storage and transfer facilities. To receive permission to construct a plant must undergo a review to show that it will not cause a violation of the Ambient Air Quality Standards(Peters, 2003).
Methods
There are two major types of pollutants that are released into the air, particulates and and gaseous pollutants. Particulates can be removed with mechanical forces while gaseous pollutants typically need to removed by chemical or physical means (Peters, 2003).
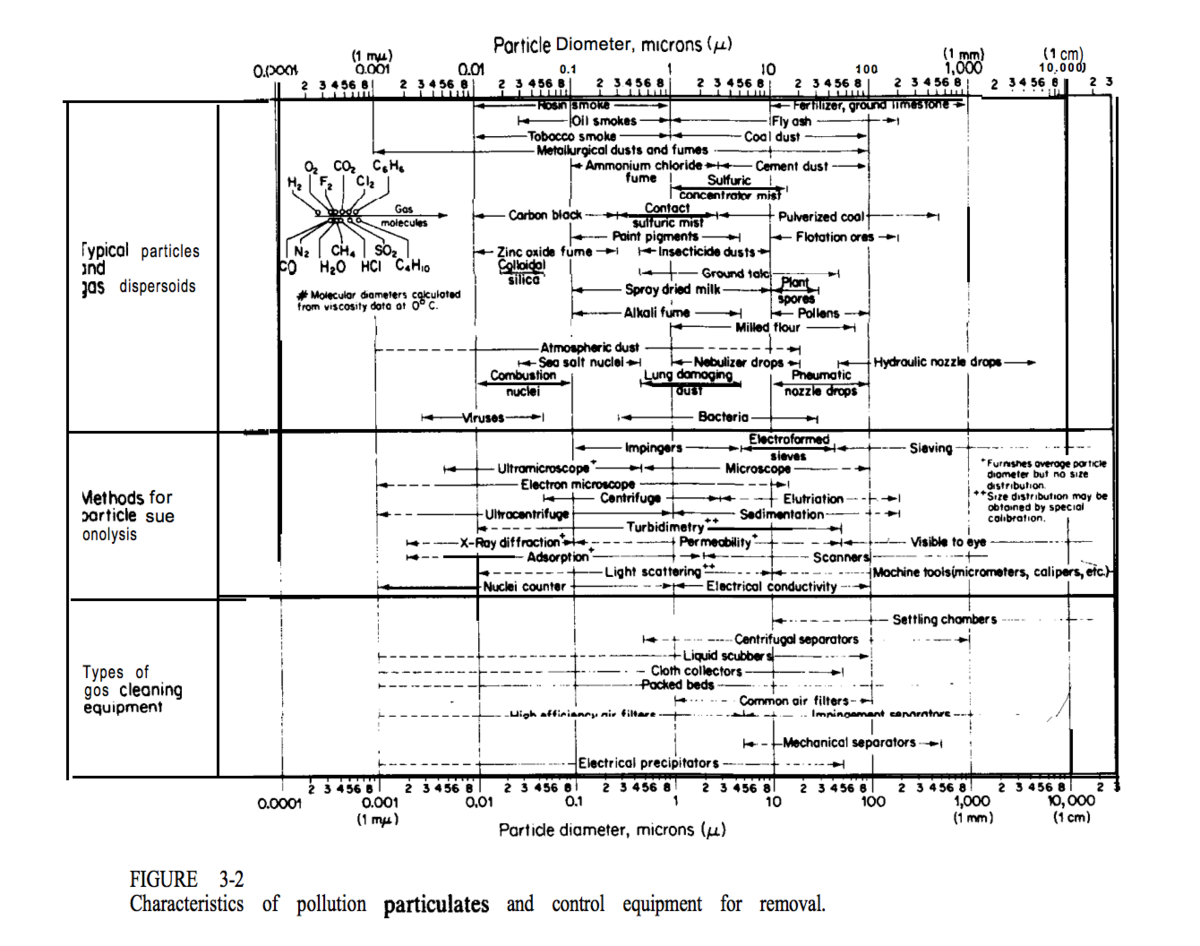
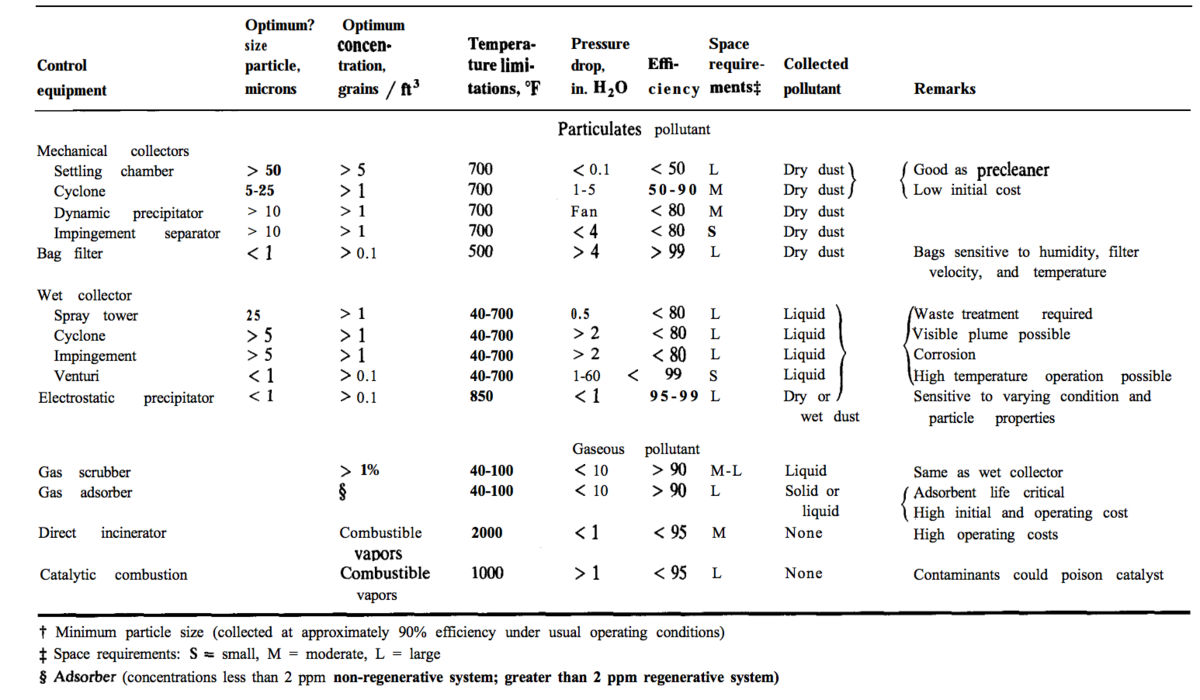
Particulates and volatile pollutants that need to be removed before disposal may be present. Particle removal equipment includes: cyclones, wet scrubbers, electrostatic precipitators, and fabric-filter systems (Seider 2009) such as bag filters (. The two charts below are from Plant Design and Economics for Chemical Engineers and show the types of equipment, separation methods, and particle sizes in different pollutant separation technologies (Peters, 1991).
For more information regarding some of these separations eqipment see Solids-involved equipment
For more information Regarding Cyclones see Cyclones, and for their design in HYSYS see Solids-involved equipment#HYSYS Simulation
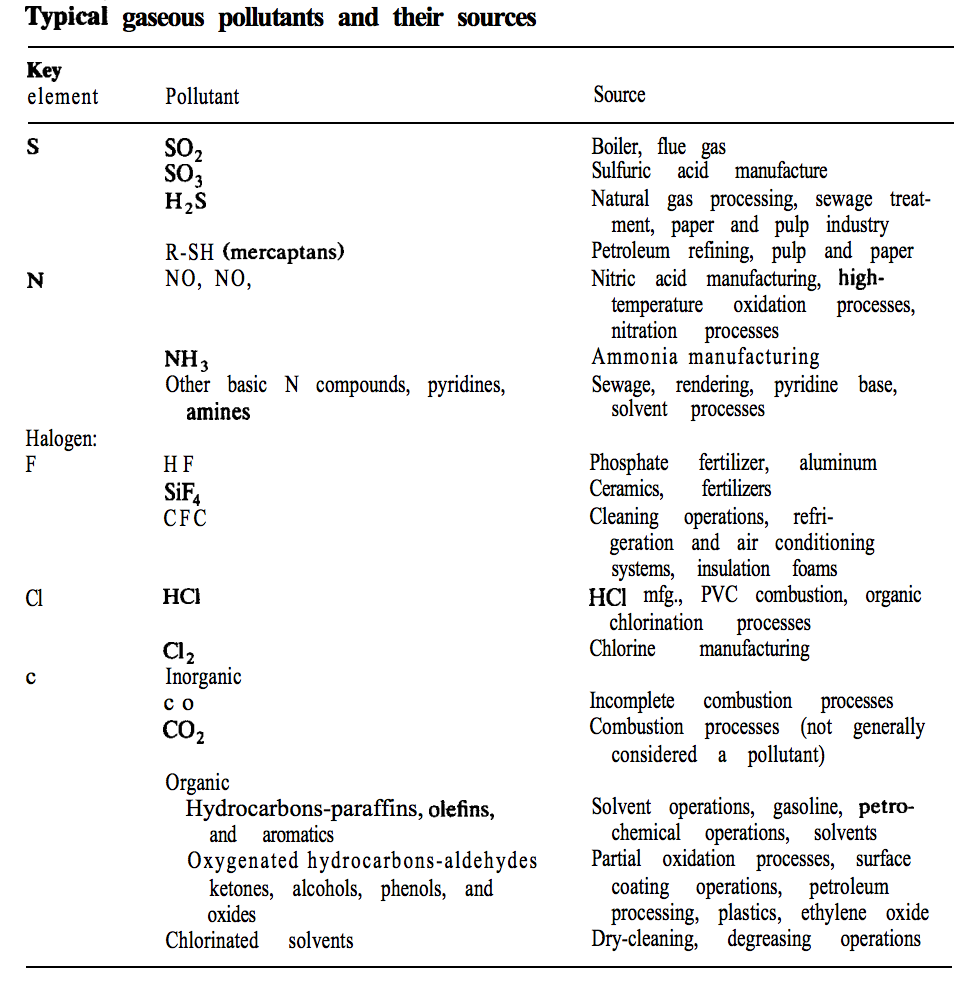
Methods for removing inorganic and organic gaseous pollutants include: absorption, adsorption, condensation, and combustion (Seider 2009 pg 609). A list of typical of gases pollutants and their sources from Plant Design and Economics for Chemical Engineers is shown below (Peters, 1991).
Typically Gas-liquid absorption processes are done completed in a vertical, countercurrent, flow through packed, plate, or spray towers. These systems require good liquid-gas contact and proper equipment. These systems also often have significant energy consumption because of large pressure drops (Peters, 2003). For high volume systems absorption by scrubbing with water or another solvent is the most widely used method (Towler, 2012). Dry adsorbents can be used to remove the last races of gaseous pollutants. Adsorption typically requires blowers, condensers, separators, and controls. You also typically need two packed beds so that one can be used while the other is regenerated. Examples of adsorbents are molecular sieves and activated carbon. Incineration is typically used when there are gas streams that have no recovery value. This can be done with direct flame or catalytic oxidation. Catalytic oxidation usually has higher capital costs, but lower operating costs because it does not require fuel.
You can find more information on Absorption and Adsorption in Separation processes
Outcomes
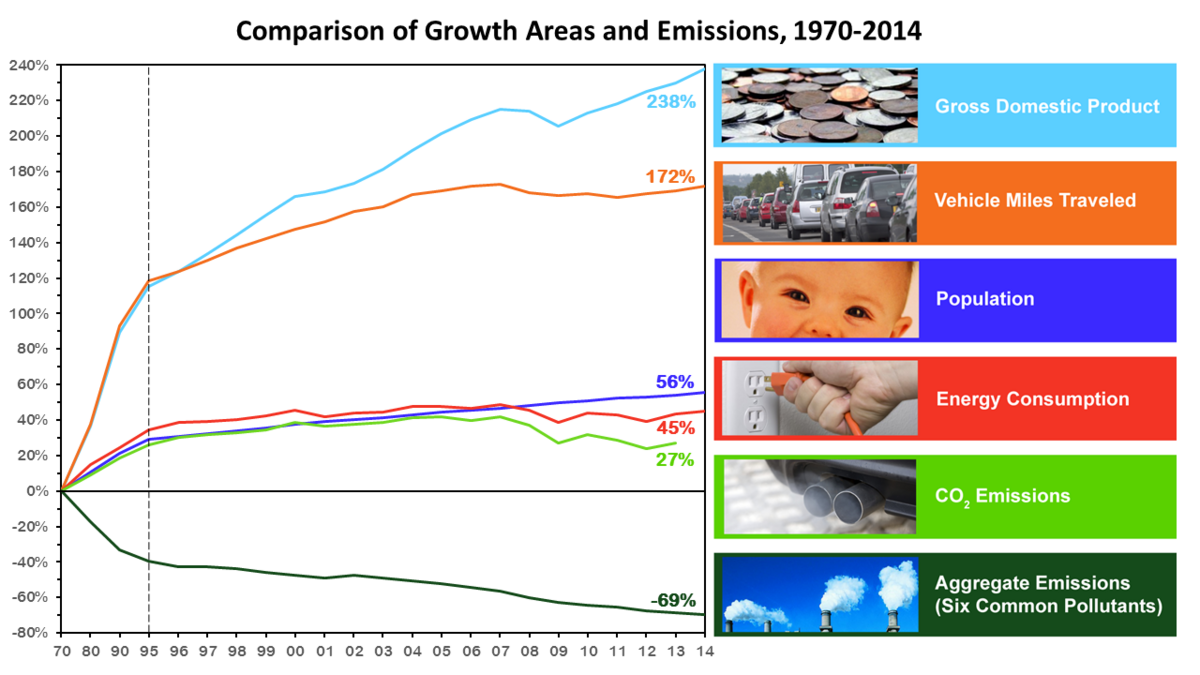
The United States implemented the Clean Air Act in 1970 and since then emissions in the U.S. have been drastically reduced. As a matter of fact despite increases in the population in the last 35 years the amount of pollutants emitted have decreased by almost 70% (EPA). More importantly this demonstrates that reasonable efforts can be put towards environmental protection without causing too much harm to industry.
Solid Waste
U.S. federal regulations require that solid waste be classified as hazardous or nonhazardous. Conditions for a classification of hazardous include: ignitability, corrosivity, reactivity, toxicity, or posing a substantial threat to the surrounding environment and its inhabitants. Hazardous waste must be treated on- or near-site before being removed in containers. Non-hazardous waste may be landfilled or incinerated in some cases. A typical estimate of costs for waste disposal is $0.03/lb for nonhazardous solids and $0.10/lb for hazardous solids. (Seider 2009 pg 609)
References
- Biegler LT, Grossmann IE, Westerberg AW. Systematic Methods of Chemical Process Design Prentice-Hall: Upper Saddle River, 1997.
- Duke Energy Company (2013). How Do Coal Fired Plants Work? Charlotte: Duke Energy.
- Lehman B, Worrell E. Electricity Production from Natural Gas Pressure Recovery Using Expansion Turbines. Lawrence Berkeley National Laboratory; 2001.
- "Overview of the Clean Air Act and Air Pollution." Environmental Protection Agency. November 17, 2015. http://www.epa.gov/clean-air-act-overview. Accessed February 5, 2016.
- Peters, Max S.; Timmerhaus, Klaus D.; West, Ronald E. (2003). "Plant Design and Economics for Chemical Engineers." McGraw Hill Higher Education.
- Seider, Seader, Lewin, Widagdo. (2009). Plant Design and Economics for Chemical Engineers, 5th Edition. Hoboken: Wiley.
- Seider, Seader, Lewin. (2008). Product and Process Design Principles, 2nd Edition. Hoboken: Wiley.
- Summary of the Clean Water Act. United States EPA website. http://www.epa.gov/laws-regulations/summary-clean-water-act
- Towler, G.P. and Sinnot, R. (2012). Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. Elsevier.
- Turton R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz J.A.; Bhattacharyya D. (2012). Analysis, Synthesis, and Design of Chemical Processes. Upper Saddle River: Prentice Hall.
- G.D. Ulrich, A Guide to Chemical Engineering Process Design and Economics, Wiley: New York, 1984.